Prion Protein Devoid of the Octapeptide Repeat Region Delays Bovine Spongiform Encephalopathy Pathogenesis in Mice
- PMID: 29046443
- PMCID: PMC5730770
- DOI: 10.1128/JVI.01368-17
Prion Protein Devoid of the Octapeptide Repeat Region Delays Bovine Spongiform Encephalopathy Pathogenesis in Mice
Abstract
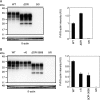
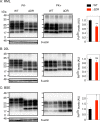
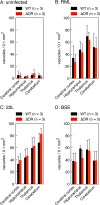
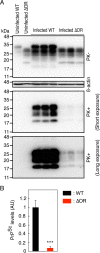
Conformational conversion of the cellular isoform of prion protein, PrPC, into the abnormally folded, amyloidogenic isoform, PrPSc, is a key pathogenic event in prion diseases, including Creutzfeldt-Jakob disease in humans and scrapie and bovine spongiform encephalopathy (BSE) in animals. We previously reported that the octapeptide repeat (OR) region could be dispensable for converting PrPC into PrPSc after infection with RML prions. We demonstrated that mice transgenically expressing mouse PrP with deletion of the OR region on the PrP knockout background, designated Tg(PrPΔOR)/Prnp0/0 mice, did not show reduced susceptibility to RML scrapie prions, with abundant accumulation of PrPScΔOR in their brains. We show here that Tg(PrPΔOR)/Prnp0/0 mice were highly resistant to BSE prions, developing the disease with markedly elongated incubation times after infection with BSE prions. The conversion of PrPΔOR into PrPScΔOR was markedly delayed in their brains. These results suggest that the OR region may have a crucial role in the conversion of PrPC into PrPSc after infection with BSE prions. However, Tg(PrPΔOR)/Prnp0/0 mice remained susceptible to RML and 22L scrapie prions, developing the disease without elongated incubation times after infection with RML and 22L prions. PrPScΔOR accumulated only slightly less in the brains of RML- or 22L-infected Tg(PrPΔOR)/Prnp0/0 mice than PrPSc in control wild-type mice. Taken together, these results indicate that the OR region of PrPC could play a differential role in the pathogenesis of BSE prions and RML or 22L scrapie prions.IMPORTANCE Structure-function relationship studies of PrPC conformational conversion into PrPSc are worthwhile to understand the mechanism of the conversion of PrPC into PrPSc We show here that, by inoculating Tg(PrPΔOR)/Prnp0/0 mice with the three different strains of RML, 22L, and BSE prions, the OR region could play a differential role in the conversion of PrPC into PrPSc after infection with RML or 22L scrapie prions and BSE prions. PrPΔOR was efficiently converted into PrPScΔOR after infection with RML and 22L prions. However, the conversion of PrPΔOR into PrPScΔOR was markedly delayed after infection with BSE prions. Further investigation into the role of the OR region in the conversion of PrPC into PrPSc after infection with BSE prions might be helpful for understanding the pathogenesis of BSE prions.
Keywords: BSE; bovine spongiform encephalopathy; octapeptide repeat; prion; prion protein; scrapie.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Strain-Dependent Prion Infection in Mice Expressing Prion Protein with Deletion of Central Residues 91-106.Int J Mol Sci. 2020 Oct 1;21(19):7260. doi: 10.3390/ijms21197260. Int J Mol Sci. 2020. PMID: 33019549 Free PMC article.
-
Central residues in prion protein PrPC are crucial for its conversion into the pathogenic isoform.J Biol Chem. 2022 Sep;298(9):102381. doi: 10.1016/j.jbc.2022.102381. Epub 2022 Aug 13. J Biol Chem. 2022. PMID: 35973512 Free PMC article.
-
Mouse-hamster chimeric prion protein (PrP) devoid of N-terminal residues 23-88 restores susceptibility to 22L prions, but not to RML prions in PrP-knockout mice.PLoS One. 2014 Oct 16;9(10):e109737. doi: 10.1371/journal.pone.0109737. eCollection 2014. PLoS One. 2014. PMID: 25330286 Free PMC article.
-
Transgenic models of prion disease.Arch Virol Suppl. 2000;(16):113-24. doi: 10.1007/978-3-7091-6308-5_10. Arch Virol Suppl. 2000. PMID: 11214913 Review.
-
The Ninth Datta Lecture. Molecular biology of transmissible spongiform encephalopathies.FEBS Lett. 1996 Jun 24;389(1):3-11. doi: 10.1016/0014-5793(96)00610-2. FEBS Lett. 1996. PMID: 8682199 Review.
Cited by
-
Virus Infection, Genetic Mutations, and Prion Infection in Prion Protein Conversion.Int J Mol Sci. 2021 Nov 18;22(22):12439. doi: 10.3390/ijms222212439. Int J Mol Sci. 2021. PMID: 34830321 Free PMC article. Review.
-
The manifold role of octapeptide repeats in prion protein assembly.Pept Sci (Hoboken). 2023 Mar;115(2):e24303. doi: 10.1002/pep2.24303. Epub 2023 Jan 30. Pept Sci (Hoboken). 2023. PMID: 37153755 Free PMC article.
-
Involvement of N- and C-terminal region of recombinant cervid prion protein in its reactivity to CWD and atypical BSE prions in real-time quaking-induced conversion reaction in the presence of high concentrations of tissue homogenates.Prion. 2020 Dec;14(1):283-295. doi: 10.1080/19336896.2020.1858694. Prion. 2020. PMID: 33345717 Free PMC article.
-
N-Terminal Regions of Prion Protein: Functions and Roles in Prion Diseases.Int J Mol Sci. 2020 Aug 28;21(17):6233. doi: 10.3390/ijms21176233. Int J Mol Sci. 2020. PMID: 32872280 Free PMC article. Review.
-
Case Report: Genetic Creutzfeldt-Jakob Disease With a G114V Mutation and One Octapeptide Repeat Deletion as a Mimic of Frontotemporal Dementia.Front Neurol. 2022 Jun 24;13:888309. doi: 10.3389/fneur.2022.888309. eCollection 2022. Front Neurol. 2022. PMID: 35812092 Free PMC article.
References
-
- Prusiner SB, Groth D, Serban A, Koehler R, Foster D, Torchia M, Burton D, Yang SL, DeArmond SJ. 1993. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc Natl Acad Sci U S A 90:10608–10612. doi:10.1073/pnas.90.22.10608. - DOI - PMC - PubMed
-
- Manson JC, Clarke AR, McBride PA, McConnell I, Hope J. 1994. PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration 3:331–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

