Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain
- PMID: 29042554
- PMCID: PMC5645389
- DOI: 10.1038/s41467-017-00952-3
Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain
Abstract
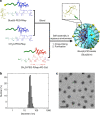
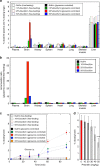
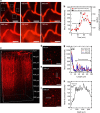
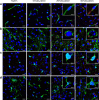
Recently, nanocarriers that transport bioactive substances to a target site in the body have attracted considerable attention and undergone rapid progression in terms of the state of the art. However, few nanocarriers can enter the brain via a systemic route through the blood-brain barrier (BBB) to efficiently reach neurons. Here we prepare a self-assembled supramolecular nanocarrier with a surface featuring properly configured glucose. The BBB crossing and brain accumulation of this nanocarrier are boosted by the rapid glycaemic increase after fasting and by the putative phenomenon of the highly expressed glucose transporter-1 (GLUT1) in brain capillary endothelial cells migrating from the luminal to the abluminal plasma membrane. The precisely controlled glucose density on the surface of the nanocarrier enables the regulation of its distribution within the brain, and thus is successfully optimized to increase the number of nanocarriers accumulating in neurons.There are only a few examples of nanocarriers that can transport bioactive substances across the blood-brain barrier. Here the authors show that by rapid glycaemic increase the accumulation of a glucosylated nanocarrier in the brain can be controlled.
Conflict of interest statement
T.Yo. and K.K. are scientific advisors of Braizon Therapeutics, Inc. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier.Angew Chem Int Ed Engl. 2020 May 18;59(21):8173-8180. doi: 10.1002/anie.201914751. Epub 2020 Mar 6. Angew Chem Int Ed Engl. 2020. PMID: 31995252 Free PMC article.
-
Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma.ACS Nano. 2018 May 22;12(5):4105-4115. doi: 10.1021/acsnano.7b06969. Epub 2018 Apr 4. ACS Nano. 2018. PMID: 29608290
-
Nanoparticle transport across the blood brain barrier.Tissue Barriers. 2016 Feb 25;4(1):e1153568. doi: 10.1080/21688370.2016.1153568. eCollection 2016 Jan-Mar. Tissue Barriers. 2016. PMID: 27141426 Free PMC article. Review.
-
Accessing targeted nanoparticles to the brain: the vascular route.Curr Med Chem. 2014;21(36):4092-9. doi: 10.2174/0929867321666140716095317. Curr Med Chem. 2014. PMID: 25039779 Review.
-
Nicotine pre-exposure reduces stroke-induced glucose transporter-1 activity at the blood-brain barrier in mice.Fluids Barriers CNS. 2015 Apr 29;12:10. doi: 10.1186/s12987-015-0005-y. Fluids Barriers CNS. 2015. PMID: 25925411 Free PMC article.
Cited by
-
Challenges and advances in glioblastoma targeted therapy: the promise of drug repurposing and biomarker exploration.Front Oncol. 2024 Oct 8;14:1441460. doi: 10.3389/fonc.2024.1441460. eCollection 2024. Front Oncol. 2024. PMID: 39439947 Free PMC article. Review.
-
Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human.Fluids Barriers CNS. 2020 Jul 22;17(1):47. doi: 10.1186/s12987-020-00209-0. Fluids Barriers CNS. 2020. PMID: 32698806 Free PMC article.
-
Elevated Cellular Uptake of Succinimide- and Glucose-Modified Liposomes for Blood-Brain Barrier Transfer and Glioblastoma Therapy.Biomedicines. 2024 Sep 20;12(9):2135. doi: 10.3390/biomedicines12092135. Biomedicines. 2024. PMID: 39335648 Free PMC article.
-
Discerning the Role of Blood Brain Barrier Dysfunction in Alzheimer's Disease.Aging Dis. 2022 Oct 1;13(5):1391-1404. doi: 10.14336/AD.2022.0130-1. eCollection 2022 Oct 1. Aging Dis. 2022. PMID: 36186141 Free PMC article.
-
Blood-Brain Barrier Conquest in Glioblastoma Nanomedicine: Strategies, Clinical Advances, and Emerging Challenges.Cancers (Basel). 2024 Sep 27;16(19):3300. doi: 10.3390/cancers16193300. Cancers (Basel). 2024. PMID: 39409919 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

