Animal Toxins Providing Insights into TRPV1 Activation Mechanism
- PMID: 29035314
- PMCID: PMC5666373
- DOI: 10.3390/toxins9100326
Animal Toxins Providing Insights into TRPV1 Activation Mechanism
Abstract
Beyond providing evolutionary advantages, venoms offer unique research tools, as they were developed to target functionally important proteins and pathways. As a key pain receptor in the nociceptive pathway, transient receptor potential vanilloid 1 (TRPV1) of the TRP superfamily has been shown to be a target for several toxins, as a way of producing pain to deter predators. Importantly, TRPV1 is involved in thermoregulation, inflammation, and acute nociception. As such, toxins provide tools to understand TRPV1 activation and modulation, a critical step in advancing pain research and the development of novel analgesics. Indeed, the phytotoxin capsaicin, which is the spicy chemical in chili peppers, was invaluable in the original cloning and characterization of TRPV1. The unique properties of each subsequently characterized toxin have continued to advance our understanding of functional, structural, and biophysical characteristics of TRPV1. By building on previous reviews, this work aims to provide a comprehensive summary of the advancements made in TRPV1 research in recent years by employing animal toxins, in particular DkTx, RhTx, BmP01, Echis coloratus toxins, APHCs and HCRG21. We examine each toxin's functional aspects, behavioral effects, and structural features, all of which have contributed to our current knowledge of TRPV1. We additionally discuss the key features of TRPV1's outer pore domain, which proves to be the target of the currently discussed toxins.
Keywords: TRPV1; centipede toxin; nociception; outer pore domain; pain; scorpion toxin; sea anemone; snake toxin; spider toxin; venom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
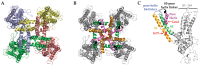
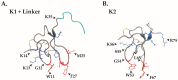
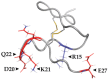

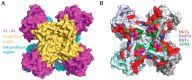
Similar articles
-
Different types of toxins targeting TRPV1 in pain.Toxicon. 2013 Sep;71:66-75. doi: 10.1016/j.toxicon.2013.05.016. Epub 2013 Jun 2. Toxicon. 2013. PMID: 23732125 Review.
-
Protein toxins of the Echis coloratus viper venom directly activate TRPV1.Biochim Biophys Acta Gen Subj. 2017 Mar;1861(3):615-623. doi: 10.1016/j.bbagen.2017.01.004. Epub 2017 Jan 4. Biochim Biophys Acta Gen Subj. 2017. PMID: 28063984
-
Painful toxins acting at TRPV1.Toxicon. 2008 Feb;51(2):163-73. doi: 10.1016/j.toxicon.2007.10.012. Epub 2007 Oct 26. Toxicon. 2008. PMID: 18061640 Review.
-
TRPV1 pore turret dictates distinct DkTx and capsaicin gating.Proc Natl Acad Sci U S A. 2018 Dec 11;115(50):E11837-E11846. doi: 10.1073/pnas.1809662115. Epub 2018 Nov 21. Proc Natl Acad Sci U S A. 2018. PMID: 30463948 Free PMC article.
-
Lethal potency in mice of toxins from scorpion, sea anemone, snake and bee venoms following intraperitoneal and intracisternal injection.Toxicon. 1984;22(2):308-11. doi: 10.1016/0041-0101(84)90032-1. Toxicon. 1984. PMID: 6145236
Cited by
-
Endothelial TRPV1 as an Emerging Molecular Target to Promote Therapeutic Angiogenesis.Cells. 2020 May 27;9(6):1341. doi: 10.3390/cells9061341. Cells. 2020. PMID: 32471282 Free PMC article. Review.
-
Venom Peptide Toxins Targeting the Outer Pore Region of Transient Receptor Potential Vanilloid 1 in Pain: Implications for Analgesic Drug Development.Int J Mol Sci. 2022 May 21;23(10):5772. doi: 10.3390/ijms23105772. Int J Mol Sci. 2022. PMID: 35628583 Free PMC article. Review.
-
An Apriori Algorithm-Based Association Analysis of Analgesic Drugs in Chinese Medicine Prescriptions Recorded From Patients With Rheumatoid Arthritis Pain.Front Pain Res (Lausanne). 2022 Jul 25;3:937259. doi: 10.3389/fpain.2022.937259. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 35959238 Free PMC article.
-
Toxins in Drug Discovery and Pharmacology.Toxins (Basel). 2018 Mar 16;10(3):126. doi: 10.3390/toxins10030126. Toxins (Basel). 2018. PMID: 29547537 Free PMC article.
-
The Transient Receptor Potential Channel Vanilloid 1 Is Critical in Innate Airway Epithelial Responses to Protease Allergens.Am J Respir Cell Mol Biol. 2020 Aug;63(2):198-208. doi: 10.1165/rcmb.2019-0170OC. Am J Respir Cell Mol Biol. 2020. PMID: 32182090 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

