Development and maintenance of the brain's immune toolkit: Microglia and non-parenchymal brain macrophages
- PMID: 29030904
- PMCID: PMC6001428
- DOI: 10.1002/dneu.22545
Development and maintenance of the brain's immune toolkit: Microglia and non-parenchymal brain macrophages
Abstract
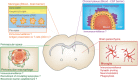
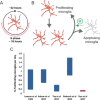
Microglia and non-parenchymal macrophages located in the perivascular space, the meninges and the choroid plexus are independent immune populations that play vital roles in brain development, homeostasis, and tissue healing. Resident macrophages account for a significant proportion of cells in the brain and their density remains stable throughout the lifespan thanks to constant turnover. Microglia develop from yolk sac progenitors, later evolving through intermediate progenitors in a fine-tuned process in which intrinsic factors and external stimuli combine to progressively sculpt their cell type-specific transcriptional profiles. Recent evidence demonstrates that non-parenchymal macrophages are also generated during early embryonic development. In recent years, the development of powerful fate mapping approaches combined with novel genomic and transcriptomic methodologies have greatly expanded our understanding of how brain macrophages develop and acquire specialized functions, and how cell population dynamics are regulated. Here, we review the transcription factors, epigenetic remodeling, and signaling pathways orchestrating the embryonic development of microglia and non-parenchymal macrophages. Next, we describe the dynamics of the macrophage populations of the brain and discuss the role of progenitor cells, to gain a better understanding of their functions in the healthy and diseased brain. © 2017 Wiley Periodicals, Inc. Develop Neurobiol 78: 561-579, 2018.
Keywords: development; lineage; microglia; progenitor; self-renewal.
© 2017 The Authors Developmental Neurobiology Published by Wiley Periodicals, Inc.
Figures

Similar articles
-
Brain Parenchymal and Extraparenchymal Macrophages in Development, Homeostasis, and Disease.J Immunol. 2020 Jan 15;204(2):294-305. doi: 10.4049/jimmunol.1900821. J Immunol. 2020. PMID: 31907272 Free PMC article. Review.
-
Distinct Features of Brain-Resident Macrophages: Microglia and Non-Parenchymal Brain Macrophages.Mol Cells. 2021 May 31;44(5):281-291. doi: 10.14348/molcells.2021.0060. Mol Cells. 2021. PMID: 33972475 Free PMC article. Review.
-
Early Fate Defines Microglia and Non-parenchymal Brain Macrophage Development.Cell. 2020 Apr 30;181(3):557-573.e18. doi: 10.1016/j.cell.2020.03.021. Epub 2020 Apr 6. Cell. 2020. PMID: 32259484
-
Microglia and macrophages in brain homeostasis and disease.Nat Rev Immunol. 2018 Apr;18(4):225-242. doi: 10.1038/nri.2017.125. Epub 2017 Nov 20. Nat Rev Immunol. 2018. PMID: 29151590 Review.
-
Diversity and function of brain-associated macrophages.Curr Opin Immunol. 2022 Jun;76:102181. doi: 10.1016/j.coi.2022.102181. Epub 2022 Apr 21. Curr Opin Immunol. 2022. PMID: 35462276 Review.
Cited by
-
Microglia: a promising therapeutic target in spinal cord injury.Neural Regen Res. 2025 Feb 1;20(2):454-463. doi: 10.4103/NRR.NRR-D-23-02044. Epub 2024 Apr 16. Neural Regen Res. 2025. PMID: 38819048 Free PMC article.
-
Effects of Ischemic Stroke on Interstitial Fluid Clearance in Mouse Brain: a Bead Study.Cell Mol Neurobiol. 2023 Nov;43(8):4141-4156. doi: 10.1007/s10571-023-01400-1. Epub 2023 Aug 27. Cell Mol Neurobiol. 2023. PMID: 37634198
-
Transcriptional and epigenetic regulation of microglia in maintenance of brain homeostasis and neurodegeneration.Front Mol Neurosci. 2023 Jan 9;15:1072046. doi: 10.3389/fnmol.2022.1072046. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36698776 Free PMC article. Review.
-
Trends in perivascular macrophages research from 1997 to 2021: A bibliometric analysis.CNS Neurosci Ther. 2023 Mar;29(3):816-830. doi: 10.1111/cns.14034. Epub 2022 Dec 13. CNS Neurosci Ther. 2023. PMID: 36514189 Free PMC article.
-
Microglia in Circumventricular Organs: The Pineal Gland Example.ASN Neuro. 2022 Jan-Dec;14:17590914221135697. doi: 10.1177/17590914221135697. ASN Neuro. 2022. PMID: 36317305 Free PMC article. Review.
References
-
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. 2007. Local self‐renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10:1538–1543. - PubMed
-
- Alliot F, Godin I, Pessac B. 1999. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117:145–152. - PubMed
-
- Amit I, Winter DR, Jung S. 2016. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol 17:18–25. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

