The role of hepatocyte growth factor in corneal wound healing
- PMID: 29024692
- PMCID: PMC5831200
- DOI: 10.1016/j.exer.2017.10.006
The role of hepatocyte growth factor in corneal wound healing
Abstract
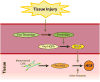
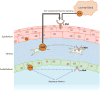
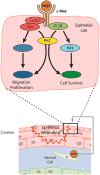
Hepatocyte growth factor (HGF) is a glycoprotein produced by mesenchymal cells and operates as a key molecule for tissue generation and renewal. During corneal injury, HGF is primarily secreted by stromal fibroblasts and promotes epithelial wound healing in a paracrine manner. While this mesenchymal-epithelial interaction is well characterized in various organs and the cornea, the role of HGF in corneal stromal and endothelial wound healing is understudied. In addition, HGF has been shown to play an anti-fibrotic role by inhibiting myofibroblast generation and subsequent production of a disorganized extracellular matrix and tissue fibrosis. Therefore, HGF represents a potential therapeutic tool in numerous organs in which myofibroblasts are responsible for tissue scarring. Corneal fibrosis can be a devastating sequela of injury and can result in corneal opacification and retrocorneal membrane formation leading to severe vision loss. In this article, we concisely review the available literature regarding the role of HGF in corneal wound healing. We highlight the influence of HGF on cellular behaviors in each corneal layer. Additionally, we suggest the possibility that HGF may represent a therapeutic tool for interrupting dysregulated corneal repair processes to improve patient outcomes.
Keywords: Fibrosis; HGF; Myofibroblast; TGF-β; Wound healing.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Interleukin-1 and Transforming Growth Factor Beta: Commonly Opposing, but Sometimes Supporting, Master Regulators of the Corneal Wound Healing Response to Injury.Invest Ophthalmol Vis Sci. 2021 Apr 1;62(4):8. doi: 10.1167/iovs.62.4.8. Invest Ophthalmol Vis Sci. 2021. PMID: 33825855 Free PMC article.
-
The corneal fibrosis response to epithelial-stromal injury.Exp Eye Res. 2016 Jan;142:110-8. doi: 10.1016/j.exer.2014.09.012. Exp Eye Res. 2016. PMID: 26675407 Free PMC article. Review.
-
Modulation of human corneal stromal cell differentiation by hepatocyte growth factor and substratum compliance.Exp Eye Res. 2018 Nov;176:235-242. doi: 10.1016/j.exer.2018.09.001. Epub 2018 Sep 5. Exp Eye Res. 2018. PMID: 30193807 Free PMC article.
-
Role of aquaporins in corneal healing post chemical injury.Exp Eye Res. 2023 Mar;228:109390. doi: 10.1016/j.exer.2023.109390. Epub 2023 Jan 22. Exp Eye Res. 2023. PMID: 36696947 Free PMC article. Review.
-
Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea.Invest Ophthalmol Vis Sci. 1993 Jul;34(8):2544-61. Invest Ophthalmol Vis Sci. 1993. PMID: 8392040
Cited by
-
Keratocyte mechanobiology.Exp Eye Res. 2020 Nov;200:108228. doi: 10.1016/j.exer.2020.108228. Epub 2020 Sep 10. Exp Eye Res. 2020. PMID: 32919993 Free PMC article. Review.
-
Interleukin-1 and Transforming Growth Factor Beta: Commonly Opposing, but Sometimes Supporting, Master Regulators of the Corneal Wound Healing Response to Injury.Invest Ophthalmol Vis Sci. 2021 Apr 1;62(4):8. doi: 10.1167/iovs.62.4.8. Invest Ophthalmol Vis Sci. 2021. PMID: 33825855 Free PMC article.
-
Sirt6 deficiency impairs corneal epithelial wound healing.Aging (Albany NY). 2018 Aug 2;10(8):1932-1946. doi: 10.18632/aging.101513. Aging (Albany NY). 2018. PMID: 30070973 Free PMC article.
-
Growth factor-eluting hydrogels for management of corneal defects.Mater Sci Eng C Mater Biol Appl. 2021 Jan;120:111790. doi: 10.1016/j.msec.2020.111790. Epub 2020 Dec 10. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33545916 Free PMC article.
-
Hepatocyte growth factor upregulates MMP1 and MMP10 expression and resolves corneal fibrosis.Sci Rep. 2024 Nov 2;14(1):26421. doi: 10.1038/s41598-024-75488-w. Sci Rep. 2024. PMID: 39488561 Free PMC article.
References
-
- Andresen JL, Ehlers N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res. 1998;17(1):79–87. - PubMed
-
- Araki-Sasaki K, Danjo S, Kawaguchi S, Hosohata J, Tano Y. Human hepatocyte growth factor (HGF) in the aqueous humor. Jpn J Ophthalmol. 1997;41(6):409–413. - PubMed
-
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8(10):404–410. - PubMed
-
- Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32(3):223–237. - PubMed
-
- Birk DE, Fitch JM, Linsenmayer TF. Organization of collagen types I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 1986;27(10):1470–1477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

