Lost in Transportation: Nucleocytoplasmic Transport Defects in ALS and Other Neurodegenerative Diseases
- PMID: 29024655
- PMCID: PMC5678982
- DOI: 10.1016/j.neuron.2017.07.029
Lost in Transportation: Nucleocytoplasmic Transport Defects in ALS and Other Neurodegenerative Diseases
Abstract
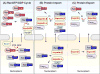
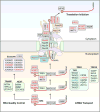
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal neurodegenerative disease characterized by degeneration of upper and lower motor neurons in the brain and spinal cord. The hallmark pathological feature in most cases of ALS is nuclear depletion and cytoplasmic accumulation of the protein TDP-43 in degenerating neurons. Consistent with this pattern of intracellular protein redistribution, impaired nucleocytoplasmic trafficking has emerged as a mechanism contributing to ALS pathology. Dysfunction in nucleocytoplasmic transport is also an emerging theme in physiological aging and other related neurodegenerative diseases, such as Huntington's and Alzheimer's diseases. Here we review transport through the nuclear pore complex, pointing out vulnerabilities that may underlie ALS and potentially contribute to this and other age-related neurodegenerative diseases.
Keywords: ALS; C9ORF72; FTD; aging; dipeptide repeats; neurodegenerative diseases; nuclear pore; nucleocytoplasmic transport.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Nuclear transport dysfunction: a common theme in amyotrophic lateral sclerosis and frontotemporal dementia.J Neurochem. 2016 Aug;138 Suppl 1:134-44. doi: 10.1111/jnc.13642. Epub 2016 Jun 15. J Neurochem. 2016. PMID: 27087014 Review.
-
TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD.Nat Neurosci. 2018 Feb;21(2):228-239. doi: 10.1038/s41593-017-0047-3. Epub 2018 Jan 8. Nat Neurosci. 2018. PMID: 29311743 Free PMC article.
-
Dipeptide repeat protein inclusions are rare in the spinal cord and almost absent from motor neurons in C9ORF72 mutant amyotrophic lateral sclerosis and are unlikely to cause their degeneration.Acta Neuropathol Commun. 2015 Jun 25;3:38. doi: 10.1186/s40478-015-0218-y. Acta Neuropathol Commun. 2015. PMID: 26108573 Free PMC article.
-
Traffic jam at the nuclear pore: All roads lead to nucleocytoplasmic transport defects in ALS/FTD.Neurobiol Dis. 2020 Jul;140:104835. doi: 10.1016/j.nbd.2020.104835. Epub 2020 Mar 14. Neurobiol Dis. 2020. PMID: 32179176 Free PMC article. Review.
-
Nuclear trafficking in amyotrophic lateral sclerosis and frontotemporal lobar degeneration.Brain. 2017 Jan;140(1):13-26. doi: 10.1093/brain/aww197. Epub 2016 Aug 6. Brain. 2017. PMID: 27497493 Review.
Cited by
-
Effects of PB-TURSO on the transcriptional and metabolic landscape of sporadic ALS fibroblasts.Ann Clin Transl Neurol. 2022 Oct;9(10):1551-1564. doi: 10.1002/acn3.51648. Epub 2022 Sep 9. Ann Clin Transl Neurol. 2022. PMID: 36083004 Free PMC article.
-
Compounds that extend longevity are protective in neurodegenerative diseases and provide a novel treatment strategy for these devastating disorders.Mech Ageing Dev. 2020 Sep;190:111297. doi: 10.1016/j.mad.2020.111297. Epub 2020 Jun 28. Mech Ageing Dev. 2020. PMID: 32610099 Free PMC article. Review.
-
Aging in a Dish: iPSC-Derived and Directly Induced Neurons for Studying Brain Aging and Age-Related Neurodegenerative Diseases.Annu Rev Genet. 2018 Nov 23;52:271-293. doi: 10.1146/annurev-genet-120417-031534. Epub 2018 Sep 12. Annu Rev Genet. 2018. PMID: 30208291 Free PMC article. Review.
-
Teaching case 3-2019: Are nuclear clefts or invaginations the niche of intranuclear inclusions in FTLD-TDP?Clin Neuropathol. 2019 May/Jun;38(3):97-99. doi: 10.5414/NP301202. Clin Neuropathol. 2019. PMID: 31023421 Free PMC article.
-
Loss of function of the ALS-associated NEK1 kinase disrupts microtubule homeostasis and nuclear import.Sci Adv. 2023 Aug 18;9(33):eadi5548. doi: 10.1126/sciadv.adi5548. Epub 2023 Aug 16. Sci Adv. 2023. PMID: 37585529 Free PMC article.
References
-
- Ayala YM, Zago P, D'Ambrogio A, Xu YF, Petrucelli L, Buratti E, Baralle FE. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

