Glutamine Addiction in Kidney Cancer Suppresses Oxidative Stress and Can Be Exploited for Real-Time Imaging
- PMID: 29021138
- PMCID: PMC5791889
- DOI: 10.1158/0008-5472.CAN-17-0930
Glutamine Addiction in Kidney Cancer Suppresses Oxidative Stress and Can Be Exploited for Real-Time Imaging
Abstract
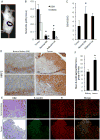
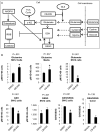
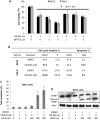
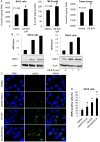
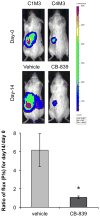
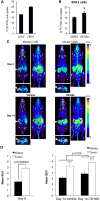
Many cancers appear to activate intrinsic antioxidant systems as a means to counteract oxidative stress. Some cancers, such as clear cell renal cell carcinoma (ccRCC), require exogenous glutamine for growth and exhibit reprogrammed glutamine metabolism, at least in part due to the glutathione pathway, an efficient cellular buffering system that counteracts reactive oxygen species and other oxidants. We show here that ccRCC xenograft tumors under the renal capsule exhibit enhanced oxidative stress compared with adjacent normal tissue and the contralateral kidney. Upon glutaminase inhibition with CB-839 or BPTES, the RCC cell lines SN12PM-6-1 (SN12) and 786-O exhibited decreased survival and pronounced apoptosis associated with a decreased GSH/GSSG ratio, augmented nuclear factor erythroid-related factor 2, and increased 8-oxo-7,8-dihydro-2'-deoxyguanosine, a marker of DNA damage. SN12 tumor xenografts showed decreased growth when treated with CB-839. Furthermore, PET imaging confirmed that ccRCC tumors exhibited increased tumoral uptake of 18F-(2S,4R)4-fluoroglutamine compared with the kidney in the orthotopic mouse model. This technique can be utilized to follow changes in ccRCC metabolism in vivo Further development of these paradigms will lead to new treatment options with glutaminase inhibitors and the utility of PET to identify and manage patients with ccRCC who are likely to respond to glutaminase inhibitors in the clinic. Cancer Res; 77(23); 6746-58. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
Similar articles
-
The glutaminase inhibitor telaglenastat enhances the antitumor activity of signal transduction inhibitors everolimus and cabozantinib in models of renal cell carcinoma.PLoS One. 2021 Nov 3;16(11):e0259241. doi: 10.1371/journal.pone.0259241. eCollection 2021. PLoS One. 2021. PMID: 34731180 Free PMC article.
-
Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice.Int J Radiat Biol. 2019 Apr;95(4):436-442. doi: 10.1080/09553002.2018.1558299. Epub 2019 Jan 15. Int J Radiat Biol. 2019. PMID: 30557074 Free PMC article.
-
PET/MR Imaging of a Lung Metastasis Model of Clear Cell Renal Cell Carcinoma with (2S,4R)-4-[18F]Fluoroglutamine.Mol Imaging Biol. 2022 Dec;24(6):959-972. doi: 10.1007/s11307-022-01747-9. Epub 2022 Jun 22. Mol Imaging Biol. 2022. PMID: 35732988 Free PMC article.
-
Enhancing the Efficacy of Glutamine Metabolism Inhibitors in Cancer Therapy.Trends Cancer. 2021 Aug;7(8):790-804. doi: 10.1016/j.trecan.2021.04.003. Epub 2021 May 18. Trends Cancer. 2021. PMID: 34020912 Free PMC article. Review.
-
Recent Development of Small Molecule Glutaminase Inhibitors.Curr Top Med Chem. 2018;18(6):432-443. doi: 10.2174/1568026618666180525100830. Curr Top Med Chem. 2018. PMID: 29793408 Review.
Cited by
-
Preclinical evidence of the enhanced effectiveness of combined rapamycin and AICAR in reducing kidney cancer.Mol Oncol. 2018 Nov;12(11):1917-1934. doi: 10.1002/1878-0261.12370. Epub 2018 Oct 12. Mol Oncol. 2018. PMID: 30107094 Free PMC article.
-
Efficacy of Dual Inhibition of Glycolysis and Glutaminolysis for Therapy of Renal Lesions in Tsc2+/- Mice.Neoplasia. 2019 Feb;21(2):230-238. doi: 10.1016/j.neo.2018.12.003. Epub 2019 Jan 8. Neoplasia. 2019. PMID: 30622053 Free PMC article.
-
Gamma-Glutamyltransferase 1 Promotes Clear Cell Renal Cell Carcinoma Initiation and Progression.Mol Cancer Res. 2019 Sep;17(9):1881-1892. doi: 10.1158/1541-7786.MCR-18-1204. Epub 2019 May 31. Mol Cancer Res. 2019. PMID: 31151999 Free PMC article.
-
Downregulation of Glutamine Synthetase, not glutaminolysis, is responsible for glutamine addiction in Notch1-driven acute lymphoblastic leukemia.Mol Oncol. 2021 May;15(5):1412-1431. doi: 10.1002/1878-0261.12877. Epub 2021 Feb 13. Mol Oncol. 2021. PMID: 33314742 Free PMC article.
-
Metabolic Adaptation as Potential Target in Papillary Renal Cell Carcinomas Based on Their In Situ Metabolic Characteristics.Int J Mol Sci. 2022 Sep 13;23(18):10587. doi: 10.3390/ijms231810587. Int J Mol Sci. 2022. PMID: 36142502 Free PMC article.
References
-
- Day AM, Brown JD, Taylor SR, Rand JD, Morgan BA, Veal EA. Inactivation of a peroxiredoxin by hydrogen peroxide is critical for thioredoxin-mediated repair of oxidized proteins and cell survival. Molecular cell. 2012;45:398–408. - PubMed
-
- Wu Q, Ni X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Current drug targets. 2015;16:13–9. - PubMed
-
- Bielski BH, Arudi RL, Sutherland MW. A study of the reactivity of HO2/O2- with unsaturated fatty acids. The Journal of biological chemistry. 1983;258:4759–61. - PubMed
-
- Finkel T. Redox-dependent signal transduction. FEBS letters. 2000;476:52–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

