Human amnion-derived mesenchymal stem cells promote osteogenic and angiogenic differentiation of human adipose-derived stem cells
- PMID: 29020045
- PMCID: PMC5636128
- DOI: 10.1371/journal.pone.0186253
Human amnion-derived mesenchymal stem cells promote osteogenic and angiogenic differentiation of human adipose-derived stem cells
Abstract
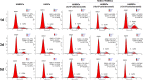
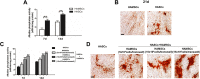
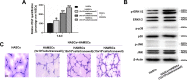
Tissue engineering using suitable mesenchymal stem cells (MSCs) shows great potential to regenerate bone defects. Our previous studies have indicated that human amnion-derived mesenchymal stem cells (HAMSCs) could promote the osteogenic differentiation of human bone marrow mesenchymal stem cells (HBMSCs). Human adipose-derived stem cells (HASCs), obtained from adipose tissue in abundance, are capable of multi-lineage differentiation. In this study, the effects of HAMSCs on osteogenic and angiogenic differentiation of HASCs were systematically investigated. Proliferation levels were measured by flow cytometry. Osteoblastic differentiation and mineralization were investigated using chromogenic alkaline phosphatase activity (ALP) activity substrate assays, Alizarin red S staining, real-time polymerase chain reaction (real-time PCR) analysis of osteogenic marker expression, and Western blotting. We found that HAMSCs increased the proliferation and osteoblastic differentiation of HASCs. Moreover, enzyme-linked immunosorbent assay (ELISA) and human umbilical vein endothelial cells (HUVECs) tube formation suggested HAMSCs enhanced angiogenic potential of HASCs via secretion of increased vascular endothelial growth factor (VEGF). Thus, we conclude that HAMSC might be a valuable therapeutic approach to promote HASCs-involved bone regeneration.
Conflict of interest statement
Figures

Similar articles
-
Human amnion mesenchymal stem cells promote proliferation and osteogenic differentiation in human bone marrow mesenchymal stem cells.J Mol Histol. 2015 Feb;46(1):13-20. doi: 10.1007/s10735-014-9600-5. Epub 2014 Nov 29. J Mol Histol. 2015. PMID: 25432786
-
Amniotic Mesenchymal Stromal Cells Exhibit Preferential Osteogenic and Chondrogenic Differentiation and Enhanced Matrix Production Compared With Adipose Mesenchymal Stromal Cells.Am J Sports Med. 2017 Sep;45(11):2637-2646. doi: 10.1177/0363546517706138. Epub 2017 May 25. Am J Sports Med. 2017. PMID: 28541092 Free PMC article.
-
Human amnion-derived mesenchymal stem cells enhance the osteogenic differentiation of human adipose-derived stem cells by promoting adiponectin excretion via the APPL1-ERK1/2 signaling pathway.IUBMB Life. 2020 Feb;72(2):296-304. doi: 10.1002/iub.2165. Epub 2019 Sep 11. IUBMB Life. 2020. PMID: 31509344
-
Impact of Age on Human Adipose Stem Cells for Bone Tissue Engineering.Cell Transplant. 2017 Sep;26(9):1496-1504. doi: 10.1177/0963689717721203. Cell Transplant. 2017. PMID: 29113460 Free PMC article. Review.
-
Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: Their comparative efficacies and synergistic effects.J Biomed Mater Res A. 2017 Sep;105(9):2640-2648. doi: 10.1002/jbm.a.36089. Epub 2017 May 17. J Biomed Mater Res A. 2017. PMID: 28419760 Review.
Cited by
-
The angiogenic properties of human amniotic membrane stem cells are enhanced in gestational diabetes and associate with fetal adiposity.Stem Cell Res Ther. 2021 Dec 20;12(1):608. doi: 10.1186/s13287-021-02678-y. Stem Cell Res Ther. 2021. PMID: 34930438 Free PMC article.
-
Osteogenic Differentiation Effect of BMP-9 with Phenamil and Simvastatin on Intact Human Amniotic Epithelial Stem Cells.Iran Biomed J. 2022 Nov 1;26(6):463-74. doi: 10.52547/ibj.3748. Iran Biomed J. 2022. PMID: 36437797 Free PMC article.
-
Stem/progenitor cells in fetuses and newborns: overview of immunohistochemical markers.Cell Regen. 2021 Jul 5;10(1):22. doi: 10.1186/s13619-021-00084-6. Cell Regen. 2021. PMID: 34219203 Free PMC article. Review.
-
Human amniotic mesenchymal stem cells to promote/suppress cancer: two sides of the same coin.Stem Cell Res Ther. 2021 Feb 12;12(1):126. doi: 10.1186/s13287-021-02196-x. Stem Cell Res Ther. 2021. PMID: 33579346 Free PMC article. Review.
-
Human Amniotic Mesenchymal Stem Cells Promote Endogenous Bone Regeneration.Front Endocrinol (Lausanne). 2020 Oct 2;11:543623. doi: 10.3389/fendo.2020.543623. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33133012 Free PMC article. Review.
References
-
- Llambes F, Silvestre FJ, Caffesse R. Vertical guided bone regeneration with bioabsorbable barriers. Journal of periodontology. 2007;78(10):2036–42. . - PubMed
-
- Maeda H, Tomokiyo A, Fujii S, Wada N, Akamine A. Promise of periodontal ligament stem cells in regeneration of periodontium. Stem cell research & therapy. 2011;2(4):33 doi: 10.1186/scrt74 ; PubMed Central PMCID: PMC3219064. - DOI - PMC - PubMed
-
- Park SH, Wang HL. Clinical significance of incision location on guided bone regeneration: human study. Journal of periodontology. 2007;78(1):47–51. doi: 10.1902/jop.2007.060125 . - DOI - PubMed
-
- Petite H, Viateau V, Bensaid W, Meunier A, de Pollak C, Bourguignon M, et al. Tissue-engineered bone regeneration. Nature biotechnology. 2000;18(9):959–63. doi: 10.1038/79449 . - DOI - PubMed
-
- Boccaccini AR, Blaker JJ. Bioactive composite materials for tissue engineering scaffolds. Expert review of medical devices. 2005;2(3):303–17. doi: 10.1586/17434440.2.3.303 . - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

