Intermittent Ethanol during Adolescence Leads to Lasting Behavioral Changes in Adulthood and Alters Gene Expression and Histone Methylation in the PFC
- PMID: 29018328
- PMCID: PMC5622951
- DOI: 10.3389/fnmol.2017.00307
Intermittent Ethanol during Adolescence Leads to Lasting Behavioral Changes in Adulthood and Alters Gene Expression and Histone Methylation in the PFC
Abstract
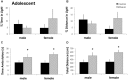
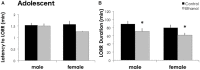
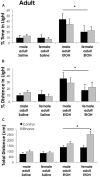
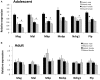
Adolescents primarily consume alcohol in binges, which can be particularly harmful to the developing frontal cortex and increase risk for an adult alcohol use disorder. We conducted a study investigating immediate and long lasting changes to the prefrontal cortex (PFC) transcriptome to determine the molecular mechanisms underlying adult ethanol behavioral sensitivity following binge ethanol in adolescence. DBA/2J mice were orally dosed with 4 g/kg ethanol intermittently from day 29 to 42. Adolescent mice were tested for anxiety-like behavior and ethanol sensitivity using the loss of righting reflex task. As adults, mice were tested for cognitive changes using the novel object recognition task, ethanol-induced anxiolysis and ethanol sensitivity. Adolescent binge ethanol altered ethanol sensitivity in young mice and led to lasting memory deficits in the object recognition test and greater ethanol sensitivity in adulthood. Using genomic profiling of transcripts in the PFC, we found that binge ethanol reduced myelin-related gene expression and altered chromatin modifying genes involved in histone demethylation at H3K9 and H3K36. We hypothesize that ethanol's actions on histone methylation may be a switch for future transcriptional changes that underlie the behavioral changes lasting into adulthood.
Keywords: adolescent; epigenetics; ethanol; genomics; prefrontal cortex.
Figures


Similar articles
-
Adolescent social housing protects against adult emotional and cognitive deficits and alters the PFC and NAc transcriptome in male and female C57BL/6J mice.Front Neurosci. 2023 Dec 7;17:1287584. doi: 10.3389/fnins.2023.1287584. eCollection 2023. Front Neurosci. 2023. PMID: 38130694 Free PMC article.
-
Adolescent binge ethanol impacts H3K36me3 regulation of synaptic genes.Front Mol Neurosci. 2023 Mar 3;16:1082104. doi: 10.3389/fnmol.2023.1082104. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36937047 Free PMC article.
-
Alcohol Consumption during Adolescence in a Mouse Model of Binge Drinking Alters the Intrinsic Excitability and Function of the Prefrontal Cortex through a Reduction in the Hyperpolarization-Activated Cation Current.J Neurosci. 2018 Jul 4;38(27):6207-6222. doi: 10.1523/JNEUROSCI.0550-18.2018. Epub 2018 Jun 18. J Neurosci. 2018. PMID: 29915134 Free PMC article.
-
Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence?Physiol Behav. 2015 Sep 1;148:122-30. doi: 10.1016/j.physbeh.2015.01.027. Epub 2015 Jan 23. Physiol Behav. 2015. PMID: 25624108 Free PMC article. Review.
-
Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking.Alcohol Alcohol. 2014 Mar-Apr;49(2):187-92. doi: 10.1093/alcalc/agt164. Epub 2013 Nov 10. Alcohol Alcohol. 2014. PMID: 24217958 Review.
Cited by
-
Cholinergic REST-G9a gene repression through HMGB1-TLR4 neuroimmune signaling regulates basal forebrain cholinergic neuron phenotype.Front Mol Neurosci. 2022 Aug 22;15:992627. doi: 10.3389/fnmol.2022.992627. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36072299 Free PMC article.
-
Neuropharmacology of Alcohol Addiction with Special Emphasis on Proteomic Approaches for Identification of Novel Therapeutic Targets.Curr Neuropharmacol. 2023;21(1):119-132. doi: 10.2174/1570159X20666220811092906. Curr Neuropharmacol. 2023. PMID: 35959616 Free PMC article.
-
Bisphenol a Exposure in Utero Disrupts Hypothalamic Gene Expression Particularly Genes Suspected in Autism Spectrum Disorders and Neuron and Hormone Signaling.Int J Mol Sci. 2020 Apr 29;21(9):3129. doi: 10.3390/ijms21093129. Int J Mol Sci. 2020. PMID: 32365465 Free PMC article.
-
Adolescent social housing protects against adult emotional and cognitive deficits and alters the PFC and NAc transcriptome in male and female C57BL/6J mice.Front Neurosci. 2023 Dec 7;17:1287584. doi: 10.3389/fnins.2023.1287584. eCollection 2023. Front Neurosci. 2023. PMID: 38130694 Free PMC article.
-
Adolescent alcohol and nicotine exposure alters the adult response to alcohol use.Adv Drug Alcohol Res. 2023 Nov 22;3:11880. doi: 10.3389/adar.2023.11880. eCollection 2023. Adv Drug Alcohol Res. 2023. PMID: 38389816 Free PMC article. Review.
References
-
- Barbier E., Johnstone A. L., Khomtchouk B. B., Tapocik J. D., Pitcairn C., Rehman F., et al. (2016). Dependence-induced increase of alcohol self-administration and compulsive drinking mediated by the histone methyltransferase PRDM2. Mol. Psychiatry 10.1038/mp.2016.131 [Epub ahead of print]. - DOI - PMC - PubMed
-
- Beaudet G., Valable S., Bourgine J., Lelong-Boulouard V., Lanfumey L., Freret T., et al. (2016). Long-lasting effects of chronic intermittent alcohol exposure in adolescent mice on object recognition and hippocampal neuronal activity. Alcohol. Clin. Exp. Res. 40 2591–2603. 10.1111/acer.13256 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

