iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury
- PMID: 28993834
- PMCID: PMC11105708
- DOI: 10.1007/s00018-017-2676-9
iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury
Abstract
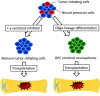
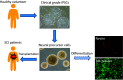
A number of studies have demonstrated that transplantation of neural precursor cells (NPCs) promotes functional recovery after spinal cord injury (SCI). However, the NPCs had been mostly harvested from embryonic stem cells or fetal tissue, raising the ethical concern. Yamanaka and his colleagues established induced pluripotent stem cells (iPSCs) which could be generated from somatic cells, and this innovative development has made rapid progression in the field of SCI regeneration. We and other groups succeeded in producing NPCs from iPSCs, and demonstrated beneficial effects after transplantation for animal models of SCI. In particular, efficacy of human iPSC-NPCs in non-human primate SCI models fostered momentum of clinical application for SCI patients. At the same time, however, artificial induction methods in iPSC technology created alternative issues including genetic and epigenetic abnormalities, and tumorigenicity after transplantation. To overcome these problems, it is critically important to select origins of somatic cells, use integration-free system during transfection of reprogramming factors, and thoroughly investigate the characteristics of iPSC-NPCs with respect to quality management. Moreover, since most of the previous studies have focused on subacute phase of SCI, establishment of effective NPC transplantation should be evaluated for chronic phase hereafter. Our group is currently preparing clinical-grade human iPSC-NPCs, and will move forward toward clinical study for subacute SCI patients soon in the near future.
Keywords: Central nervous system; Mechanisms for functional recovery; Regeneration; Safety issue; Stem cell graft.
Figures
Similar articles
-
Applications of induced pluripotent stem cell technologies in spinal cord injury.J Neurochem. 2017 Jun;141(6):848-860. doi: 10.1111/jnc.13986. Epub 2017 Apr 5. J Neurochem. 2017. PMID: 28199003 Review.
-
Human neural progenitors derived from integration-free iPSCs for SCI therapy.Stem Cell Res. 2017 Mar;19:55-64. doi: 10.1016/j.scr.2017.01.004. Epub 2017 Jan 5. Stem Cell Res. 2017. PMID: 28073086 Free PMC article.
-
Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells.Mol Brain. 2020 Sep 3;13(1):120. doi: 10.1186/s13041-020-00662-w. Mol Brain. 2020. PMID: 32883317 Free PMC article.
-
Transplanted Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Do Not Promote Functional Recovery of Pharmacologically Immunosuppressed Mice With Contusion Spinal Cord Injury.Cell Transplant. 2015;24(9):1799-812. doi: 10.3727/096368914X684079. Epub 2014 Sep 8. Cell Transplant. 2015. PMID: 25203632
-
Recent Progress in the Regeneration of Spinal Cord Injuries by Induced Pluripotent Stem Cells.Int J Mol Sci. 2019 Aug 6;20(15):3838. doi: 10.3390/ijms20153838. Int J Mol Sci. 2019. PMID: 31390782 Free PMC article. Review.
Cited by
-
Employing Endogenous NSCs to Promote Recovery of Spinal Cord Injury.Stem Cells Int. 2019 May 5;2019:1958631. doi: 10.1155/2019/1958631. eCollection 2019. Stem Cells Int. 2019. PMID: 31191666 Free PMC article. Review.
-
Regenerative medicine strategies for chronic complete spinal cord injury.Neural Regen Res. 2024 Apr;19(4):818-824. doi: 10.4103/1673-5374.382230. Neural Regen Res. 2024. PMID: 37843217 Free PMC article. Review.
-
Chemical compound-based direct reprogramming for future clinical applications.Biosci Rep. 2018 May 8;38(3):BSR20171650. doi: 10.1042/BSR20171650. Print 2018 Jun 29. Biosci Rep. 2018. PMID: 29739872 Free PMC article. Review.
-
Effects of a neurokinin-1 receptor antagonist in the acute phase after thoracic spinal cord injury in a rat model.Front Mol Neurosci. 2023 May 12;16:1128545. doi: 10.3389/fnmol.2023.1128545. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37251648 Free PMC article.
-
Generation and Evaluation of Isogenic iPSC as a Source of Cell Replacement Therapies in Patients with Kearns Sayre Syndrome.Cells. 2021 Mar 5;10(3):568. doi: 10.3390/cells10030568. Cells. 2021. PMID: 33807701 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

