CAR-T cell therapy in ovarian cancer: from the bench to the bedside
- PMID: 28969098
- PMCID: PMC5610030
- DOI: 10.18632/oncotarget.19929
CAR-T cell therapy in ovarian cancer: from the bench to the bedside
Abstract
Ovarian cancer (OC) is the most lethal gynecological malignancy and is responsible for most gynecological cancer deaths. Apart from conventional surgery, chemotherapy, and radiotherapy, chimeric antigen receptor-modified T (CAR-T) cells as a representative of adoptive cellular immunotherapy have received considerable attention in the research field of cancer treatment. CARs combine antigen specificity and T-cell-activating properties in a single fusion molecule. Several preclinical experiments and clinical trials have confirmed that adoptive cell immunotherapy using typical CAR-engineered T cells for OC is a promising treatment approach with striking clinical efficacy; moreover, the emerging CAR-Ts targeting various antigens also exert great potential. However, such therapies have side effects and toxicities, such as cytokine-associated and "on-target, off-tumor" toxicities. In this review, we systematically detail and highlight the present knowledge of CAR-Ts including the constructions, vectors, clinical applications, development challenges, and solutions of CAR-T-cell therapy for OC. We hope to provide new insight into OC treatment for the future.
Keywords: T lymphocyte; chimeric antigen receptor; immunotherapy; ovarian neoplasms; toxicity.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest relevant to this article is reported.
Figures
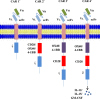
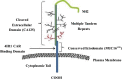
Similar articles
-
Therapeutic Potential of T Cell Chimeric Antigen Receptors (CARs) in Cancer Treatment: Counteracting Off-Tumor Toxicities for Safe CAR T Cell Therapy.Annu Rev Pharmacol Toxicol. 2016;56:59-83. doi: 10.1146/annurev-pharmtox-010814-124844. Annu Rev Pharmacol Toxicol. 2016. PMID: 26738472 Review.
-
Advances Of Chimeric Antigen Receptor T Cell Therapy In Ovarian Cancer.Onco Targets Ther. 2019 Sep 30;12:8015-8022. doi: 10.2147/OTT.S203550. eCollection 2019. Onco Targets Ther. 2019. PMID: 31686857 Free PMC article.
-
Construction of CAR-T cells targeting TM4SF1 and its anti-tumor capacity in ovarian cancer.Immunol Lett. 2023 Mar;255:1-9. doi: 10.1016/j.imlet.2023.01.011. Epub 2023 Feb 3. Immunol Lett. 2023. PMID: 36739093
-
T cells expressing VHH-directed oligoclonal chimeric HER2 antigen receptors: towards tumor-directed oligoclonal T cell therapy.Biochim Biophys Acta. 2014 Jan;1840(1):378-86. doi: 10.1016/j.bbagen.2013.09.029. Epub 2013 Sep 27. Biochim Biophys Acta. 2014. PMID: 24076235
-
Research advances in chimeric antigen receptor-modified T-cell therapy (Review).Exp Ther Med. 2021 May;21(5):484. doi: 10.3892/etm.2021.9915. Epub 2021 Mar 16. Exp Ther Med. 2021. PMID: 33790993 Free PMC article. Review.
Cited by
-
Nanotechnology and Immunotherapy in Ovarian Cancer: Tracing New Landscapes.J Pharmacol Exp Ther. 2019 Sep;370(3):636-646. doi: 10.1124/jpet.118.254979. Epub 2019 Feb 8. J Pharmacol Exp Ther. 2019. PMID: 30737357 Free PMC article. Review.
-
Exploring the clinical value of tumor microenvironment in platinum-resistant ovarian cancer.Semin Cancer Biol. 2021 Dec;77:83-98. doi: 10.1016/j.semcancer.2020.12.024. Epub 2021 Jan 18. Semin Cancer Biol. 2021. PMID: 33476723 Free PMC article. Review.
-
Epithelial Ovarian Cancer and the Immune System: Biology, Interactions, Challenges and Potential Advances for Immunotherapy.J Clin Med. 2020 Sep 14;9(9):2967. doi: 10.3390/jcm9092967. J Clin Med. 2020. PMID: 32937961 Free PMC article. Review.
-
The challenge of selecting tumor antigens for chimeric antigen receptor T-cell therapy in ovarian cancer.Med Oncol. 2022 Sep 29;39(12):232. doi: 10.1007/s12032-022-01824-7. Med Oncol. 2022. PMID: 36175774 Review.
-
Enhancing CAR-T Cell Therapy with Functional Nucleic Acids.ACS Pharmacol Transl Sci. 2021 Nov 17;4(6):1716-1727. doi: 10.1021/acsptsci.1c00188. eCollection 2021 Dec 10. ACS Pharmacol Transl Sci. 2021. PMID: 34927006 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Dinh P, Harnett P, Piccart-Gebhart MJ, Awada A. New therapies for ovarian cancer: cytotoxics and molecularly targeted agents. Crit Rev Oncol Hematol. 2008;67:103–12. - PubMed
-
- Bookman MA. Standard treatment in advanced ovarian cancer in 2005: the state of the art. Int J Gynecol Cancer. 2005;15:212–20. - PubMed
-
- Rubin SC, Randall TC, Armstrong KA, Chi DS, Hoskins WJ. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol. 1999;93:21–4. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

