Venezuelan Equine Encephalitis Virus Capsid-The Clever Caper
- PMID: 28961161
- PMCID: PMC5691631
- DOI: 10.3390/v9100279
Venezuelan Equine Encephalitis Virus Capsid-The Clever Caper
Abstract
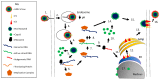
Venezuelan equine encephalitis virus (VEEV) is a New World alphavirus that is vectored by mosquitos and cycled in rodents. It can cause disease in equines and humans characterized by a febrile illness that may progress into encephalitis. Like the capsid protein of other viruses, VEEV capsid is an abundant structural protein that binds to the viral RNA and interacts with the membrane-bound glycoproteins. It also has protease activity, allowing cleavage of itself from the growing structural polypeptide during translation. However, VEEV capsid protein has additional nonstructural roles within the host cell functioning as the primary virulence factor for VEEV. VEEV capsid inhibits host transcription and blocks nuclear import in mammalian cells, at least partially due to its complexing with the host CRM1 and importin α/β1 nuclear transport proteins. VEEV capsid also shuttles between the nucleus and cytoplasm and is susceptible to inhibitors of nuclear trafficking, making it a promising antiviral target. Herein, the role of VEEV capsid in viral replication and pathogenesis will be discussed including a comparison to proteins of other alphaviruses.
Keywords: CRM1; VEEV; capsid; eastern equine encephalitis virus; importin; western equine encephalitis virus.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication.Antiviral Res. 2013 Dec;100(3):662-72. doi: 10.1016/j.antiviral.2013.10.004. Epub 2013 Oct 22. Antiviral Res. 2013. PMID: 24161512
-
Venezuelan equine encephalitis virus capsid protein inhibits nuclear import in Mammalian but not in mosquito cells.J Virol. 2008 Apr;82(8):4028-41. doi: 10.1128/JVI.02330-07. Epub 2008 Feb 6. J Virol. 2008. PMID: 18256144 Free PMC article.
-
Selective Inhibitor of Nuclear Export (SINE) Compounds Alter New World Alphavirus Capsid Localization and Reduce Viral Replication in Mammalian Cells.PLoS Negl Trop Dis. 2016 Nov 30;10(11):e0005122. doi: 10.1371/journal.pntd.0005122. eCollection 2016 Nov. PLoS Negl Trop Dis. 2016. PMID: 27902702 Free PMC article.
-
Current Understanding of the Molecular Basis of Venezuelan Equine Encephalitis Virus Pathogenesis and Vaccine Development.Viruses. 2019 Feb 18;11(2):164. doi: 10.3390/v11020164. Viruses. 2019. PMID: 30781656 Free PMC article. Review.
-
Understanding host responses to equine encephalitis virus infection: implications for therapeutic development.Expert Rev Anti Infect Ther. 2022 Dec;20(12):1551-1566. doi: 10.1080/14787210.2022.2141224. Epub 2022 Nov 4. Expert Rev Anti Infect Ther. 2022. PMID: 36305549 Review.
Cited by
-
Competitive Binding of Viral Nuclear Localization Signal Peptide and Inhibitor Ligands to Importin-α Nuclear Transport Protein.J Chem Inf Model. 2024 Jul 8;64(13):5262-5272. doi: 10.1021/acs.jcim.4c00626. Epub 2024 Jun 13. J Chem Inf Model. 2024. PMID: 38869471 Free PMC article.
-
Arthritogenic Alphavirus Capsid Protein.Life (Basel). 2021 Mar 11;11(3):230. doi: 10.3390/life11030230. Life (Basel). 2021. PMID: 33799673 Free PMC article. Review.
-
Venezuelan Equine Encephalitis Virus V3526 Vaccine RNA-Dependent RNA Polymerase Mutants Increase Vaccine Safety Through Restricted Tissue Tropism in a Murine Model.Zoonoses (Burlingt). 2022;2:2. doi: 10.15212/zoonoses-2021-0016. Epub 2022 Jan 13. Zoonoses (Burlingt). 2022. PMID: 35262074 Free PMC article.
-
Epidemic Alphaviruses: Ecology, Emergence and Outbreaks.Microorganisms. 2020 Aug 1;8(8):1167. doi: 10.3390/microorganisms8081167. Microorganisms. 2020. PMID: 32752150 Free PMC article. Review.
-
Mitochondrial-Directed Antioxidant Reduces Microglial-Induced Inflammation in Murine In Vitro Model of TC-83 Infection.Viruses. 2018 Nov 2;10(11):606. doi: 10.3390/v10110606. Viruses. 2018. PMID: 30400156 Free PMC article.
References
-
- Powers A.M., Roehrig J.T. Alphaviruses. Methods Mol. Biol. 2011;665:17–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

