The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease
- PMID: 28957666
- PMCID: PMC5657612
- DOI: 10.1016/j.neuron.2017.07.030
The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease
Abstract
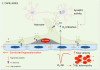
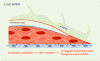
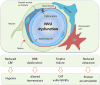
The concept of the neurovascular unit (NVU), formalized at the 2001 Stroke Progress Review Group meeting of the National Institute of Neurological Disorders and Stroke, emphasizes the intimate relationship between the brain and its vessels. Since then, the NVU has attracted the interest of the neuroscience community, resulting in considerable advances in the field. Here the current state of knowledge of the NVU will be assessed, focusing on one of its most vital roles: the coupling between neural activity and blood flow. The evidence supports a conceptual shift in the mechanisms of neurovascular coupling, from a unidimensional process involving neuronal-astrocytic signaling to local blood vessels to a multidimensional one in which mediators released from multiple cells engage distinct signaling pathways and effector systems across the entire cerebrovascular network in a highly orchestrated manner. The recently appreciated NVU dysfunction in neurodegenerative diseases, although still poorly understood, supports emerging concepts that maintaining neurovascular health promotes brain health.
Keywords: astrocytes; cerebral blood flow; endothelium; functional hyperemia; neurodegeneration; neuroimaging; pericytes; smooth muscle cells.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: An aging effect.Ageing Res Rev. 2017 Mar;34:77-87. doi: 10.1016/j.arr.2016.09.006. Epub 2016 Sep 30. Ageing Res Rev. 2017. PMID: 27697546 Free PMC article. Review.
-
The neurovascular unit in brain function and disease.Acta Physiol (Oxf). 2011 Sep;203(1):47-59. doi: 10.1111/j.1748-1716.2011.02256.x. Epub 2011 Mar 22. Acta Physiol (Oxf). 2011. PMID: 21272266 Review.
-
Neurovascular dysfunction in dementia - human cellular models and molecular mechanisms.Clin Sci (Lond). 2018 Feb 14;132(3):399-418. doi: 10.1042/CS20160720. Print 2018 Feb 14. Clin Sci (Lond). 2018. PMID: 29444850 Review.
-
Neurovascular-neuroenergetic coupling axis in the brain: master regulation by nitric oxide and consequences in aging and neurodegeneration.Free Radic Biol Med. 2017 Jul;108:668-682. doi: 10.1016/j.freeradbiomed.2017.04.026. Epub 2017 Apr 20. Free Radic Biol Med. 2017. PMID: 28435052 Review.
-
The neurovascular unit - concept review.Acta Physiol (Oxf). 2014 Apr;210(4):790-8. doi: 10.1111/apha.12250. Acta Physiol (Oxf). 2014. PMID: 24629161 Review.
Cited by
-
Wavelet and time-based cerebral autoregulation analysis using diffuse correlation spectroscopy on adults undergoing extracorporeal membrane oxygenation therapy.PLoS One. 2024 Oct 29;19(10):e0299752. doi: 10.1371/journal.pone.0299752. eCollection 2024. PLoS One. 2024. PMID: 39471182 Free PMC article.
-
The Physiological Mechanisms of Transcranial Direct Current Stimulation to Enhance Motor Performance: A Narrative Review.Biology (Basel). 2024 Oct 2;13(10):790. doi: 10.3390/biology13100790. Biology (Basel). 2024. PMID: 39452099 Free PMC article. Review.
-
The Cerebrovascular Side of Plasticity: Microvascular Architecture across Health and Neurodegenerative and Vascular Diseases.Brain Sci. 2024 Sep 28;14(10):983. doi: 10.3390/brainsci14100983. Brain Sci. 2024. PMID: 39451997 Free PMC article. Review.
-
Brain Metabolism in Health and Neurodegeneration: The Interplay Among Neurons and Astrocytes.Cells. 2024 Oct 17;13(20):1714. doi: 10.3390/cells13201714. Cells. 2024. PMID: 39451233 Free PMC article. Review.
-
Increased Resting-State BOLD Turnover (TBOLD) is Associated With Decreased Cognitive Performance During Aging.Neurosci Insights. 2024 Oct 21;19:26331055241292592. doi: 10.1177/26331055241292592. eCollection 2024. Neurosci Insights. 2024. PMID: 39439907 Free PMC article.
References
-
- Abrahams S, Goldstein LH, Kew JJ, Brooks DJ, Lloyd CM, Frith CD, Leigh PN. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain. 1996;119(Pt 6):2105–2120. - PubMed
-
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. - DOI - PubMed
-
- Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron. 2017;94:581–594.e5. doi: 10.1016/j.neuron.2017.03.043. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

