An Envelope-Modified Tetravalent Dengue Virus-Like-Particle Vaccine Has Implications for Flavivirus Vaccine Design
- PMID: 28956764
- PMCID: PMC5686733
- DOI: 10.1128/JVI.01181-17
An Envelope-Modified Tetravalent Dengue Virus-Like-Particle Vaccine Has Implications for Flavivirus Vaccine Design
Abstract
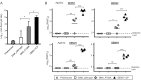
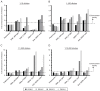
Dengue viruses (DENV) infect 50 to 100 million people each year. The spread of DENV-associated infections is one of the most serious public health problems worldwide, as there is no widely available vaccine or specific therapeutic for DENV infections. To address this, we developed a novel tetravalent dengue vaccine by utilizing virus-like particles (VLPs). We created recombinant DENV1 to -4 (DENV1-4) VLPs by coexpressing precursor membrane (prM) and envelope (E) proteins, with an F108A mutation in the fusion loop structure of E to increase the production of VLPs in mammalian cells. Immunization with DENV1-4 VLPs as individual, monovalent vaccines elicited strong neutralization activity against each DENV serotype in mice. For use as a tetravalent vaccine, DENV1-4 VLPs elicited high levels of neutralization activity against all four serotypes simultaneously. The neutralization antibody responses induced by the VLPs were significantly higher than those with DNA or recombinant E protein immunization. Moreover, antibody-dependent enhancement (ADE) was not observed against any serotype at a 1:10 serum dilution. We also demonstrated that the Zika virus (ZIKV) VLP production level was enhanced by introducing the same F108A mutation into the ZIKV envelope protein. Taken together, these results suggest that our strategy for DENV VLP production is applicable to other flavivirus VLP vaccine development, due to the similarity in viral structures, and they describe the promising development of an effective tetravalent vaccine against the prevalent flavivirus.IMPORTANCE Dengue virus poses one of the most serious public health problems worldwide, and the incidence of diseases caused by the virus has increased dramatically. Despite decades of effort, there is no effective treatment against dengue. A safe and potent vaccine against dengue is still needed. We developed a novel tetravalent dengue vaccine by using virus-like particles (VLPs), which are noninfectious because they lack the viral genome. Previous attempts of other groups to use dengue VLPs resulted in generally poor yields. We found that a critical amino acid mutation in the envelope protein enhances the production of VLPs. Our tetravalent vaccine elicited potent neutralizing antibody responses against all four DENV serotypes. Our findings can also be applied to vaccine development against other flaviviruses, such as Zika virus or West Nile virus.
Keywords: DNA vaccine; VLP; Zika virus; dengue virus; flavivirus; neutralizing antibodies; vaccine; virus-like particle.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: immunological properties.BMC Microbiol. 2014 Dec 18;14:233. doi: 10.1186/s12866-014-0233-3. BMC Microbiol. 2014. PMID: 25520151 Free PMC article.
-
Development of Virus-Like-Particle Vaccine and Reporter Assay for Zika Virus.J Virol. 2017 Sep 27;91(20):e00834-17. doi: 10.1128/JVI.00834-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28794019 Free PMC article.
-
A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice.PLoS Negl Trop Dis. 2018 Jan 8;12(1):e0006191. doi: 10.1371/journal.pntd.0006191. eCollection 2018 Jan. PLoS Negl Trop Dis. 2018. PMID: 29309412 Free PMC article.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
-
Humoral cross-reactivity between Zika and dengue viruses: implications for protection and pathology.Emerg Microbes Infect. 2017 May 10;6(5):e33. doi: 10.1038/emi.2017.42. Emerg Microbes Infect. 2017. PMID: 28487557 Free PMC article. Review.
Cited by
-
Virus-Like Particle Systems for Vaccine Development against Viruses in the Flaviviridae Family.Vaccines (Basel). 2019 Sep 20;7(4):123. doi: 10.3390/vaccines7040123. Vaccines (Basel). 2019. PMID: 31547131 Free PMC article. Review.
-
A Dengue Virus Serotype 1 mRNA-LNP Vaccine Elicits Protective Immune Responses.J Virol. 2021 May 24;95(12):e02482-20. doi: 10.1128/JVI.02482-20. Print 2021 May 24. J Virol. 2021. PMID: 33762420 Free PMC article.
-
In Vivo Assembly of Nanoparticles Achieved through Synergy of Structure-Based Protein Engineering and Synthetic DNA Generates Enhanced Adaptive Immunity.Adv Sci (Weinh). 2020 Feb 27;7(8):1902802. doi: 10.1002/advs.201902802. eCollection 2020 Apr. Adv Sci (Weinh). 2020. PMID: 32328416 Free PMC article.
-
Envelope domain III E324, E351, and E380 mutations lever adaptive evolution of DENV-1 genotype I.J Virol. 2024 Oct 22;98(10):e0118324. doi: 10.1128/jvi.01183-24. Epub 2024 Sep 4. J Virol. 2024. PMID: 39230303
-
Low-temperature culture enhances production of flavivirus virus-like particles in mammalian cells.Appl Microbiol Biotechnol. 2024 Feb 28;108(1):242. doi: 10.1007/s00253-024-13064-y. Appl Microbiol Biotechnol. 2024. PMID: 38416210 Free PMC article.
References
-
- WHO. 2012. Global strategy for dengue prevention and control. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/75303/1/9789241504034_eng.pdf?ua=1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

