Novel calcium-related targets of insulin in hippocampal neurons
- PMID: 28939258
- PMCID: PMC5653445
- DOI: 10.1016/j.neuroscience.2017.09.019
Novel calcium-related targets of insulin in hippocampal neurons
Abstract
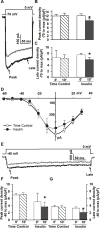
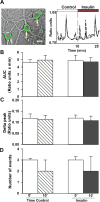
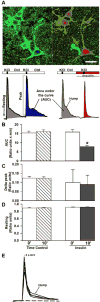
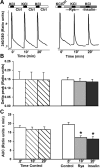
Both insulin signaling disruption and Ca2+ dysregulation are closely related to memory loss during aging and increase the vulnerability to Alzheimer's disease (AD). In hippocampal neurons, aging-related changes in calcium regulatory pathways have been shown to lead to higher intracellular calcium levels and an increase in the Ca2+-dependent afterhyperpolarization (AHP), which is associated with cognitive decline. Recent studies suggest that insulin reduces the Ca2+-dependent AHP. Given the sensitivity of neurons to insulin and evidence that brain insulin signaling is reduced with age, insulin-mediated alterations in calcium homeostasis may underlie the beneficial actions of insulin in the brain. Indeed, increasing insulin signaling in the brain via intranasal delivery has yielded promising results such as improving memory in both clinical and animal studies. However, while several mechanisms have been proposed, few have focused on regulation on intracellular Ca2+. In the present study, we further examined the effects of acute insulin on calcium pathways in primary hippocampal neurons in culture. Using the whole-cell patch-clamp technique, we found that acute insulin delivery reduced voltage-gated calcium currents. Fura-2 imaging was used to also address acute insulin effects on spontaneous and depolarization-mediated Ca2+ transients. Results indicate that insulin reduced Ca2+ transients, which appears to have involved a reduction in ryanodine receptor function. Together, these results suggest insulin regulates pathways that control intracellular Ca2+ which may reduce the AHP and improve memory. This may be one mechanism contributing to improved memory recall in response to intranasal insulin therapy in the clinic.
Keywords: aging; diabetes; electrophysiology; excitability; imaging; intranasal.
Copyright © 2017 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Disrupting function of FK506-binding protein 1b/12.6 induces the Ca²+-dysregulation aging phenotype in hippocampal neurons.J Neurosci. 2011 Feb 2;31(5):1693-703. doi: 10.1523/JNEUROSCI.4805-10.2011. J Neurosci. 2011. PMID: 21289178 Free PMC article.
-
Aging is associated with elevated intracellular calcium levels and altered calcium homeostatic mechanisms in hippocampal neurons.Neurosci Lett. 2007 May 11;418(1):77-81. doi: 10.1016/j.neulet.2007.03.005. Epub 2007 Mar 12. Neurosci Lett. 2007. PMID: 17374449 Free PMC article.
-
Reversal of Aging-Related Neuronal Ca2+ Dysregulation and Cognitive Impairment by Delivery of a Transgene Encoding FK506-Binding Protein 12.6/1b to the Hippocampus.J Neurosci. 2015 Jul 29;35(30):10878-87. doi: 10.1523/JNEUROSCI.1248-15.2015. J Neurosci. 2015. PMID: 26224869 Free PMC article.
-
Hippocampal calcium dysregulation at the nexus of diabetes and brain aging.Eur J Pharmacol. 2013 Nov 5;719(1-3):34-43. doi: 10.1016/j.ejphar.2013.07.024. Epub 2013 Jul 17. Eur J Pharmacol. 2013. PMID: 23872402 Free PMC article. Review.
-
Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store.Aging Cell. 2007 Jun;6(3):307-17. doi: 10.1111/j.1474-9726.2007.00295.x. Epub 2007 Apr 26. Aging Cell. 2007. PMID: 17465978 Free PMC article. Review.
Cited by
-
Insulin Diminishes Superoxide Increase in Cytosol and Mitochondria of Cultured Cortical Neurons Treated with Toxic Glutamate.Int J Mol Sci. 2022 Oct 20;23(20):12593. doi: 10.3390/ijms232012593. Int J Mol Sci. 2022. PMID: 36293449 Free PMC article.
-
Sensitivity of the S1 neuronal calcium network to insulin and Bay-K 8644 in vivo: Relationship to gait, motivation, and aging processes.Aging Cell. 2022 Jul;21(7):e13661. doi: 10.1111/acel.13661. Epub 2022 Jun 19. Aging Cell. 2022. PMID: 35717599 Free PMC article.
-
Brain Insulin Resistance: Focus on Insulin Receptor-Mitochondria Interactions.Life (Basel). 2021 Mar 22;11(3):262. doi: 10.3390/life11030262. Life (Basel). 2021. PMID: 33810179 Free PMC article. Review.
-
Dissecting Sex-Related Cognition between Alzheimer's Disease and Diabetes: From Molecular Mechanisms to Potential Therapeutic Strategies.Oxid Med Cell Longev. 2021 Mar 5;2021:4572471. doi: 10.1155/2021/4572471. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33747345 Free PMC article. Review.
-
Molecular elevation of insulin receptor signaling improves memory recall in aged Fischer 344 rats.Aging Cell. 2020 Oct;19(10):e13220. doi: 10.1111/acel.13220. Epub 2020 Aug 27. Aging Cell. 2020. PMID: 32852134 Free PMC article.
References
-
- Alzheimer's Association Calcium Hypothesis, W. Calcium Hypothesis of Alzheimer's disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement 2017 - PubMed
-
- Arbet-Engels C, Tartare-Deckert S, Eckhart W. C-terminal Src kinase associates with ligand-stimulated insulin-like growth factor-I receptor. J Biol Chem. 1999;274:5422–5428. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

