A Spectral Probe for Detection of Aluminum (III) Ions Using Surface Functionalized Gold Nanoparticles
- PMID: 28937661
- PMCID: PMC5666452
- DOI: 10.3390/nano7100287
A Spectral Probe for Detection of Aluminum (III) Ions Using Surface Functionalized Gold Nanoparticles
Abstract
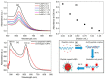
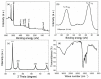
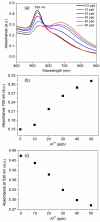
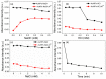
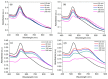
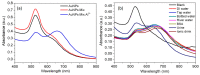
A simple green route has been developed for the synthesis of casein peptide functionalized gold nanoparticles (AuNPs), in which casein peptide acts as a reducing as well as the stabilizing agent. In this report, AuNPs have been characterized on the basis of spectroscopic and microscopic results; which showed selective and sensitive response toward Al3+ in aqueous media, and Al3+ induces aggregation of AuNPs. The sensing study performed for Al3+ revealed that the color change from red to blue was due to a red-shift in the surface plasmon resonance (SPR) band and the formation of aggregated species of AuNPs. The calibration curve determines the detection limit (LOD) for Al3+ about 20 ppb (0.067 μM) is presented using both decrease and increase in absorbance at 530 and 700 nm, respectively. This value is considerably lower than the higher limit allowed for Al3+ in drinking water by the world health organization (WHO) (7.41 μM), representing enough sensitivity to protect water quality. The intensity of the red-shifted band increases with linear pattern upon the interaction with different concentrations of Al3+, thus the possibility of producing unstable AuNPs aggregates. The method is successfully used for the detection of Al3+ in water samples collected from various sources, human urine and ionic drink. The actual response time required for AuNPs is about 1 min, this probe also have several advantages, such as ease of synthesis, functionalization and its use, high sensitivity, and enabling on-site monitoring.
Keywords: aggregation; aluminum; casein peptide; gold nanoparticles; spectrophotometer.
Conflict of interest statement
There is no conflict of interest regarding this research article.
Figures

Similar articles
-
Sunlight Induced Preparation of Functionalized Gold Nanoparticles as Recyclable Colorimetric Dual Sensor for Aluminum and Fluoride in Water.ACS Appl Mater Interfaces. 2017 May 24;9(20):17359-17368. doi: 10.1021/acsami.7b02742. Epub 2017 May 15. ACS Appl Mater Interfaces. 2017. PMID: 28470061
-
SPR responsive xylenol orange functionalized gold nanoparticles- optical sensor for estimation of Al3+ in water.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Mar 5;228:117701. doi: 10.1016/j.saa.2019.117701. Epub 2019 Oct 31. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 31759883
-
Facile synthesis of chitosan-gold nanocomposite and its application for exclusively sensitive detection of Ag+ ions.Carbohydr Polym. 2019 Dec 15;226:115290. doi: 10.1016/j.carbpol.2019.115290. Epub 2019 Sep 6. Carbohydr Polym. 2019. PMID: 31582072
-
A colorimetric probe based on 4-mercaptophenol and thioglycolic acid-functionalized gold nanoparticles for determination of phytic acid and Fe(III) ions.Mikrochim Acta. 2020 Sep 30;187(10):586. doi: 10.1007/s00604-020-04478-2. Mikrochim Acta. 2020. PMID: 32997192
-
Biogenic Synthesis of Carboxymethyl Cashew Gum Modified Gold Nanoparticles and its Sensitive and Selective Calorimetric Detection of Hg2+ Ions and Catalytic Reduction of Methyl Red.J Fluoresc. 2023 Jan;33(1):209-221. doi: 10.1007/s10895-022-03073-3. Epub 2022 Nov 18. J Fluoresc. 2023. PMID: 36399249
Cited by
-
Citrate and Polyvinylpyrrolidone Stabilized Silver Nanoparticles as Selective Colorimetric Sensor for Aluminum (III) Ions in Real Water Samples.Materials (Basel). 2020 Mar 18;13(6):1373. doi: 10.3390/ma13061373. Materials (Basel). 2020. PMID: 32197492 Free PMC article.
-
Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications.Nanomaterials (Basel). 2019 Jun 6;9(6):861. doi: 10.3390/nano9060861. Nanomaterials (Basel). 2019. PMID: 31174348 Free PMC article. Review.
-
In Situ Assembly of Nanomaterials and Molecules for the Signal Enhancement of Electrochemical Biosensors.Nanomaterials (Basel). 2021 Dec 6;11(12):3307. doi: 10.3390/nano11123307. Nanomaterials (Basel). 2021. PMID: 34947656 Free PMC article. Review.
-
Interface and Interphase in Polymer Nanocomposites with Bare and Core-Shell Gold Nanoparticles.Polymers (Basel). 2021 Feb 12;13(4):541. doi: 10.3390/polym13040541. Polymers (Basel). 2021. PMID: 33673125 Free PMC article.
-
The Peptide Functionalized Inorganic Nanoparticles for Cancer-Related Bioanalytical and Biomedical Applications.Molecules. 2021 May 27;26(11):3228. doi: 10.3390/molecules26113228. Molecules. 2021. PMID: 34072160 Free PMC article. Review.
References
-
- Mavhungu S.T., Akinlabi E.T., Onitiri M.A., Varachia F.M. Aluminum matrix composites for industrial use: advances and trends. Procedia Manuf. 2017;7:178–182. doi: 10.1016/j.promfg.2016.12.045. - DOI
-
- Valeur B., Leray I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000;205:3–40. doi: 10.1016/S0010-8545(00)00246-0. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

