Why Human Papillomaviruses Activate the DNA Damage Response (DDR) and How Cellular and Viral Replication Persists in the Presence of DDR Signaling
- PMID: 28934154
- PMCID: PMC5691620
- DOI: 10.3390/v9100268
Why Human Papillomaviruses Activate the DNA Damage Response (DDR) and How Cellular and Viral Replication Persists in the Presence of DDR Signaling
Abstract
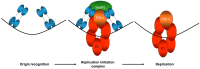
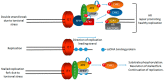
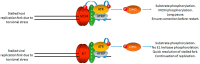
Human papillomaviruses (HPV) require the activation of the DNA damage response (DDR) in order to undergo a successful life cycle. This activation presents a challenge for the virus and the infected cell: how does viral and host replication proceed in the presence of a DDR that ordinarily arrests replication; and how do HPV16 infected cells retain the ability to proliferate in the presence of a DDR that ordinarily arrests the cell cycle? This raises a further question: why do HPV activate the DDR? The answers to these questions are only partially understood; a full understanding could identify novel therapeutic strategies to target HPV cancers. Here, we propose that the rapid replication of an 8 kb double stranded circular genome during infection creates aberrant DNA structures that attract and activate DDR proteins. Therefore, HPV replication in the presence of an active DDR is a necessity for a successful viral life cycle in order to resolve these DNA structures on viral genomes; without an active DDR, successful replication of the viral genome would not proceed. We discuss the essential role of TopBP1 in this process and also how viral and cellular replication proceeds in HPV infected cells in the presence of DDR signals.
Keywords: ATM (ataxia-telangiectasia mutated); ATR (ataxia telangiectasia and Rad3 related); DNA damage response; DNA damage signaling; E1; E2; HPV; MRN (Mre11-Rad50-Nbs1); TopBP1; cervical cancer; head and neck cancer; homologous recombination; human papillomavirus; initiation; life cycle; replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
STAT-5 Regulates Transcription of the Topoisomerase IIβ-Binding Protein 1 (TopBP1) Gene To Activate the ATR Pathway and Promote Human Papillomavirus Replication.mBio. 2015 Dec 22;6(6):e02006-15. doi: 10.1128/mBio.02006-15. mBio. 2015. PMID: 26695634 Free PMC article.
-
Modulation of the DNA damage response during the life cycle of human papillomaviruses.Virus Res. 2017 Mar 2;231:41-49. doi: 10.1016/j.virusres.2016.11.006. Epub 2016 Nov 9. Virus Res. 2017. PMID: 27836727 Free PMC article. Review.
-
FANCD2 Binds Human Papillomavirus Genomes and Associates with a Distinct Set of DNA Repair Proteins to Regulate Viral Replication.mBio. 2017 Feb 14;8(1):e02340-16. doi: 10.1128/mBio.02340-16. mBio. 2017. PMID: 28196964 Free PMC article.
-
Productive replication of human papillomavirus 31 requires DNA repair factor Nbs1.J Virol. 2014 Aug;88(15):8528-44. doi: 10.1128/JVI.00517-14. Epub 2014 May 21. J Virol. 2014. PMID: 24850735 Free PMC article.
-
Human Papillomavirus and the DNA Damage Response: Exploiting Host Repair Pathways for Viral Replication.Viruses. 2017 Aug 18;9(8):232. doi: 10.3390/v9080232. Viruses. 2017. PMID: 28820495 Free PMC article. Review.
Cited by
-
Bioinformatics Analysis of Human Papillomavirus 16 Integration in Cervical Cancer: Changes in MAGI-1 Expression in Premalignant Lesions and Invasive Carcinoma.Cancers (Basel). 2024 Jun 14;16(12):2225. doi: 10.3390/cancers16122225. Cancers (Basel). 2024. PMID: 38927930 Free PMC article.
-
A Lack of Effectiveness in the ATM-Orchestrated DNA Damage Response Contributes to the DNA Repair Defect of HPV-Positive Head and Neck Cancer Cells.Front Oncol. 2022 May 31;12:765968. doi: 10.3389/fonc.2022.765968. eCollection 2022. Front Oncol. 2022. PMID: 35719921 Free PMC article.
-
Subversion of Host Innate Immunity by Human Papillomavirus Oncoproteins.Pathogens. 2020 Apr 17;9(4):292. doi: 10.3390/pathogens9040292. Pathogens. 2020. PMID: 32316236 Free PMC article. Review.
-
High-risk human papillomavirus oncogenes disrupt the Fanconi anemia DNA repair pathway by impairing localization and de-ubiquitination of FancD2.PLoS Pathog. 2019 Feb 28;15(2):e1007442. doi: 10.1371/journal.ppat.1007442. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30818369 Free PMC article.
-
The Role of Nucleic Acid Sensing in Controlling Microbial and Autoimmune Disorders.Int Rev Cell Mol Biol. 2019;345:35-136. doi: 10.1016/bs.ircmb.2018.08.002. Epub 2018 Sep 25. Int Rev Cell Mol Biol. 2019. PMID: 30904196 Free PMC article. Review.
References
-
- Gillison M.L., Koch W.M., Capone R.B., Spafford M., Westra W.H., Wu L., Zahurak M.L., Daniel R.W., Viglione M., Symer D.E., et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

