Durability of antiretroviral therapy regimens and determinants for change in HIV-1-infected patients in the TREAT Asia HIV Observational Database (TAHOD-LITE)
- PMID: 28933705
- PMCID: PMC5862772
- DOI: 10.3851/IMP3194
Durability of antiretroviral therapy regimens and determinants for change in HIV-1-infected patients in the TREAT Asia HIV Observational Database (TAHOD-LITE)
Abstract
Background: The durability of first-line regimen is important to achieve long-term treatment success for the management of HIV infection. Our analysis describes the duration of sequential ART regimens and identifies the determinants leading to treatment change in HIV-positive patients initiating in Asia.
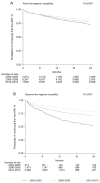
Methods: All HIV-positive adult patients initiating first-line ART in 2003-2013, from eight clinical sites among seven countries in Asia. Patient follow-up was to May 2014. Kaplan-Meier curves were used to estimate the time to second-line ART and third-line ART regimen. Factors associated with treatment durability were assessed using Cox proportional hazards model.
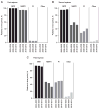
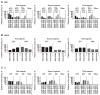
Results: A total of 16,962 patients initiated first-line ART. Of these, 4,336 patients initiated second-line ART over 38,798 person-years (pys), a crude rate of 11.2 (95% CI 10.8, 11.5) per 100 pys. The probability of being on first-line ART increased from 83.7% (95% CI 82.1, 85.1%) in 2003-2005 to 87.9% (95% CI 87.1, 88.6%) in 2010-2013. Third-line ART was initiated by 1,135 patients over 8,078 pys, a crude rate of 14.0 (95% CI 13.3, 14.9) per 100 pys. The probability of continuing second-line ART significantly increased from 64.9% (95% CI 58.5, 70.6%) in 2003-2005 to 86.2% (95% CI 84.7, 87.6%) in 2010-2013.
Conclusions: Rates of discontinuation of first- and second-line regimens have decreased over the last decade in Asia. Subsequent regimens were of shorter duration compared to the first-line regimen initiated in the same year period. Lower CD4+ T-cell count and the use of suboptimal regimens were important factors associated with higher risk of treatment switch.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures
Similar articles
-
Adverse Drug Reactions Among Patients Initiating Second-Line Antiretroviral Therapy in South Africa.Drug Saf. 2018 Dec;41(12):1343-1353. doi: 10.1007/s40264-018-0698-3. Drug Saf. 2018. PMID: 30043384 Free PMC article.
-
Hepatitis B and C Co-Infection in HIV Patients from the TREAT Asia HIV Observational Database: Analysis of Risk Factors and Survival.PLoS One. 2016 Mar 2;11(3):e0150512. doi: 10.1371/journal.pone.0150512. eCollection 2016. PLoS One. 2016. PMID: 26933963 Free PMC article.
-
Trends in mortality among ART-treated HIV-infected adults in the Asia-Pacific region between 1999 and 2017: results from the TREAT Asia HIV Observational Database (TAHOD) and Australian HIV Observational Database (AHOD) of IeDEA Asia-Pacific.J Int AIDS Soc. 2019 Jan;22(1):e25219. doi: 10.1002/jia2.25219. J Int AIDS Soc. 2019. PMID: 30615271 Free PMC article.
-
Improved survival in HIV treatment programmes in Asia.Antivir Ther. 2016;21(6):517-527. doi: 10.3851/IMP3041. Epub 2016 Feb 10. Antivir Ther. 2016. PMID: 26961354 Free PMC article.
-
Antiretroviral therapy: when and what to start-- an American perspective.Curr HIV/AIDS Rep. 2004 Jun;1(2):59-67. doi: 10.1007/s11904-004-0009-8. Curr HIV/AIDS Rep. 2004. PMID: 16091224 Review.
Cited by
-
Effects of Chinese Medicine on the Survival of AIDS Patients Administered Second-Line ART in Rural Areas of China: A Retrospective Cohort Study Based on Real-World Data.Evid Based Complement Alternat Med. 2022 Jan 27;2022:5103768. doi: 10.1155/2022/5103768. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35126600 Free PMC article.
-
Antiretroviral therapy regimen modification rates and associated factors in a cohort of HIV/AIDS patients in Asmara, Eritrea: a 16-year retrospective analysis.Sci Rep. 2023 Mar 14;13(1):4183. doi: 10.1038/s41598-023-30804-8. Sci Rep. 2023. PMID: 36918596 Free PMC article.
-
Virological failure and HIV drug resistance among adults living with HIV on second-line antiretroviral therapy in the Asia-Pacific.HIV Med. 2021 Mar;22(3):201-211. doi: 10.1111/hiv.13006. Epub 2020 Nov 5. HIV Med. 2021. PMID: 33151020 Free PMC article.
-
Trends and factors associated with modification or discontinuation of the initial antiretroviral regimen during the first year of treatment in the Turkish HIV-TR Cohort, 2011-2017.AIDS Res Ther. 2021 Jan 9;18(1):4. doi: 10.1186/s12981-020-00328-6. AIDS Res Ther. 2021. PMID: 33422112 Free PMC article.
-
Mortality Risk Factors Among People Living with HIV Receiving Second-line Antiretroviral Therapy in Rural China.Curr HIV Res. 2024;22(2):100-108. doi: 10.2174/011570162X280721240108065502. Curr HIV Res. 2024. PMID: 38310467
References
-
- Sterne JAC, Hernán MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. - PubMed
-
- Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. - PubMed
-
- Madec Y, Laureillard D, Pinoges L, et al. Response to highly active antiretroviral therapy among severely immunocompromised HIV-infected patients in Cambodia. AIDS. 2007;21:351–359. - PubMed
-
- Spacek LA, Shihab HM, Kamya MR, et al. Response to antiretroviral therapy in HIV-infected patients attending a public, urban clinic in Kampala, Uganda. Clin Infect Dis. 2006;42:252–259. - PubMed
-
- May MT, Sterne JAC, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

