Decitabine inhibits T cell proliferation via a novel TET2-dependent mechanism and exerts potent protective effect in mouse auto- and allo-immunity models
- PMID: 28915632
- PMCID: PMC5593603
- DOI: 10.18632/oncotarget.18063
Decitabine inhibits T cell proliferation via a novel TET2-dependent mechanism and exerts potent protective effect in mouse auto- and allo-immunity models
Abstract
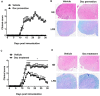
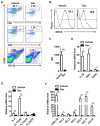
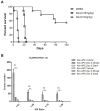
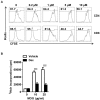
Multiple sclerosis (MS) is an autoimmune disease characterized by the dysregulated immune response including innate and adaptive immune responses. Increasing evidence has proven the importance of epigenetic modification in the progression of MS. Recent studies revealed that low-dose decitabine (Dec, 5-Aza-2'-deoxycytidine), which incorporates into replicating DNA and inhibits DNA methylation, could prevent experimental autoimmune encephalomyelitis (EAE) development by increasing the number of regulatory T cells (Tregs). Here, we showed that higher-dose decitabine relative to previous studies could also distinctly protect mice from EAE and allogeneic cardiac transplantation. Mechanistic studies revealed decitabine suppressed innate responses in EAE mice through inhibiting the activation of microglia and monocyte-derived macrophages that contributed to reduce the severity of EAE. Furthermore, differentiation of naïve CD4+ T cells into Th1 and Th17 cells was significantly suppressed by decitabine in vivo and in vitro. Though in vitro studies showed decitabine could induce Treg differentiation, there was no obvious change in the percentage of Tregs in Dec-treated EAE mice. Most importantly, we found that T cell proliferation was potently inhibited in vivo and in vitro by higher-dose decitabine through increased gene expression of the DNA dioxygenase TET2 which facilitated the expression of several cell cycle inhibitors. Collectively, our study provides novel mechanistic insights of using the epigenetic modifying agents in the management of both allo- and auto-immune responses.
Keywords: T cell proliferation; TET2; cardiac transplantation; decitabine; experimental autoimmune encephalomyelitis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures

Similar articles
-
Green tea EGCG, T cells, and T cell-mediated autoimmune diseases.Mol Aspects Med. 2012 Feb;33(1):107-18. doi: 10.1016/j.mam.2011.10.001. Epub 2011 Oct 14. Mol Aspects Med. 2012. PMID: 22020144 Review.
-
Inhibiting cardiac allograft rejection with interleukin-35 therapy combined with decitabine treatment in mice.Transpl Immunol. 2013 Dec;29(1-4):99-104. doi: 10.1016/j.trim.2013.10.001. Epub 2013 Oct 6. Transpl Immunol. 2013. PMID: 24103733
-
Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance.Brain Behav Immun. 2015 Nov;50:101-114. doi: 10.1016/j.bbi.2015.06.021. Epub 2015 Jun 27. Brain Behav Immun. 2015. PMID: 26130320
-
Dual roles of the adenosine A2a receptor in autoimmune neuroinflammation.J Neuroinflammation. 2016 Feb 26;13:48. doi: 10.1186/s12974-016-0512-z. J Neuroinflammation. 2016. PMID: 26920550 Free PMC article.
-
A Novel Cognition of Decitabine: Insights into Immunomodulation and Antiviral Effects.Molecules. 2022 Mar 18;27(6):1973. doi: 10.3390/molecules27061973. Molecules. 2022. PMID: 35335337 Free PMC article. Review.
Cited by
-
Pharmacological modulation of T cell immunity results in long-term remission of autoimmune arthritis.Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2100939118. doi: 10.1073/pnas.2100939118. Proc Natl Acad Sci U S A. 2021. PMID: 33941676 Free PMC article.
-
Effects of Treatment with the Hypomethylating Agent 5-aza-2'-deoxycytidine in Murine Type II Collagen-Induced Arthritis.Pharmaceuticals (Basel). 2019 Nov 27;12(4):174. doi: 10.3390/ph12040174. Pharmaceuticals (Basel). 2019. PMID: 31783688 Free PMC article.
-
Decitabine-Containing Conditioning Regimen for Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Intermediate- and High-Risk Myelodysplastic Syndrome/Acute Myeloid Leukemia: Potential Decrease in the Incidence of Acute Graft versus Host Disease.Cancer Manag Res. 2019 Dec 4;11:10195-10203. doi: 10.2147/CMAR.S229768. eCollection 2019. Cancer Manag Res. 2019. PMID: 31824191 Free PMC article.
-
[Effect and safety of 10-day decitabine-containing conditioning regimen for allogeneic hematopoietic stem cell transplantation in 31 patients with acute myeloid leukemia/myelodysplastic syndrome].Zhonghua Xue Ye Xue Za Zhi. 2023 Jun 14;44(6):472-478. doi: 10.3760/cma.j.issn.0253-2727.2023.06.005. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 37550202 Free PMC article. Chinese.
-
Decitabine Inhibits Gamma Delta T Cell Cytotoxicity by Promoting KIR2DL2/3 Expression.Front Immunol. 2018 Mar 26;9:617. doi: 10.3389/fimmu.2018.00617. eCollection 2018. Front Immunol. 2018. PMID: 29632540 Free PMC article.
References
-
- Yin QQ, Liu CX, Wu YL, Wu SF, Wang Y, Zhang X, Hu XJ, Pu JX, Lu Y, Zhou HC, Wang HL, Nie H, Sun HD, et al. Preventive and therapeutic effects of adenanthin on experimental autoimmune encephalomyelitis by inhibiting NF-kappaB signaling. J Immunol. 2013;191:2115–2125. doi: 10.4049/jimmunol.1203546. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

