Bioelectrospray Methodology for Dissection of the Host-pathogen Interaction in Human Tuberculosis
- PMID: 28904991
- PMCID: PMC5593078
- DOI: 10.21769/BioProtoc.2418
Bioelectrospray Methodology for Dissection of the Host-pathogen Interaction in Human Tuberculosis
Abstract
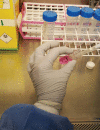
Standard cell culture models have been used to investigate disease pathology and to test new therapies for over fifty years. However, these model systems have often failed to mimic the changes occurring within three-dimensional (3-D) space where pathology occurs in vivo. To truthfully represent this, an emerging paradigm in biology is the importance of modelling disease in a physiologically relevant 3-D environment. One of the approaches for 3-D cell culture is bioelectrospray technology. This technique uses an alginate-based 3-D environment as an inert backbone within which mammalian cells and extracellular matrix can be incorporated. These alginate-based matrices produce highly reproducible results and can be mixed with different extracellular matrix components. This protocol describes a 3-D system incorporating mycobacteria, primary human blood mononuclear cells and collagen-alginate matrix to dissect the host-pathogen interaction in tuberculosis.
Keywords: Alginate-based matrices; Bioelectrospray; Collagen; Extracellular matrix; Multicellular 3-D cell culture; Tuberculosis.
Figures




Similar articles
-
Dissection of the host-pathogen interaction in human tuberculosis using a bioengineered 3-dimensional model.Elife. 2017 Jan 7;6:e21283. doi: 10.7554/eLife.21283. Elife. 2017. PMID: 28063256 Free PMC article.
-
The Extracellular Matrix Regulates Granuloma Necrosis in Tuberculosis.J Infect Dis. 2015 Aug 1;212(3):463-73. doi: 10.1093/infdis/jiv076. Epub 2015 Feb 12. J Infect Dis. 2015. PMID: 25676469 Free PMC article.
-
A Bioengineered Three-Dimensional Cell Culture Platform Integrated with Microfluidics To Address Antimicrobial Resistance in Tuberculosis.mBio. 2017 Feb 7;8(1):e02073-16. doi: 10.1128/mBio.02073-16. mBio. 2017. PMID: 28174307 Free PMC article.
-
Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery.Acc Chem Res. 2008 Jan;41(1):139-48. doi: 10.1021/ar7000827. Epub 2007 Jul 27. Acc Chem Res. 2008. PMID: 17655274 Review.
-
Modeling Host-Pathogen Interactions in the Context of the Microenvironment: Three-Dimensional Cell Culture Comes of Age.Infect Immun. 2018 Oct 25;86(11):e00282-18. doi: 10.1128/IAI.00282-18. Print 2018 Nov. Infect Immun. 2018. PMID: 30181350 Free PMC article. Review.
Cited by
-
Dissection of the host-pathogen interaction in human tuberculosis using a bioengineered 3-dimensional model.Elife. 2017 Jan 7;6:e21283. doi: 10.7554/eLife.21283. Elife. 2017. PMID: 28063256 Free PMC article.
-
Tissue-resident-like CD4+ T cells secreting IL-17 control Mycobacterium tuberculosis in the human lung.J Clin Invest. 2021 May 17;131(10):e142014. doi: 10.1172/JCI142014. J Clin Invest. 2021. PMID: 33848273 Free PMC article. Clinical Trial.
-
Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-α.Elife. 2020 Feb 24;9:e52668. doi: 10.7554/eLife.52668. Elife. 2020. PMID: 32091388 Free PMC article.
-
B cell heterogeneity in human tuberculosis highlights compartment-specific phenotype and functional roles.Commun Biol. 2024 May 16;7(1):584. doi: 10.1038/s42003-024-06282-7. Commun Biol. 2024. PMID: 38755239 Free PMC article.
-
Anti-Tuberculosis Activity of Three Carbapenems, Clofazimine and Nitazoxanide Using a Novel Ex Vivo Phenotypic Drug Susceptibility Model of Human Tuberculosis.Antibiotics (Basel). 2022 Sep 20;11(10):1274. doi: 10.3390/antibiotics11101274. Antibiotics (Basel). 2022. PMID: 36289932 Free PMC article.
References
-
- Al Shammari B., Shiomi T., Tezera L., Bielecka M. K., Workman V., Sathyamoorthy T., Mauri F., Jayasinghe S. N., Robertson B. D., D'Armiento J., Friedland J. S. and Elkington P. T.(2015). The extracellular matrix regulates granuloma necrosis in tuberculosis. J Infect Dis 212(3): 463-473. - PMC - PubMed
-
- Tezera L. B., Bielecka M. K., Chancellor A., Reichmann M. T., Shammari B. A., Brace P., Batty A., Tocheva A., Jogai S., Marshall B. G., Tebruegge M., Jayasinghe S. N., Mansour S. and Elkington P. T.(2017). Dissection of the host-pathogen interaction in human tuberculosis using a bioengineered 3-dimensional model. eLife 6:e21283. - PMC - PubMed
-
- WHO(2016). Global tuberculosis report 2016.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

