A role for BRG1 in the regulation of genes required for development of the lymphatic system
- PMID: 28903392
- PMCID: PMC5589631
- DOI: 10.18632/oncotarget.18976
A role for BRG1 in the regulation of genes required for development of the lymphatic system
Abstract
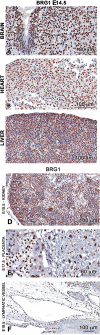
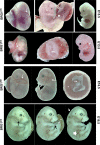
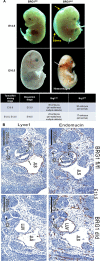
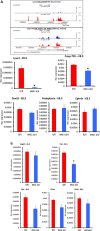
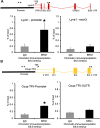
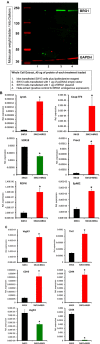
Lymphatic vasculature is an important part of the cardiovascular system with multiple functions, including regulation of the return of interstitial fluid (lymph) to the bloodstream, immune responses, and fat absorption. Consequently, lymphatic vasculature defects are involved in many pathological processes, including tumor metastasis and lymphedema. BRG1 is an important player in the developmental window when the lymphatic system is initiated. In the current study, we used tamoxifen inducible Rosa26CreERT2-BRG1floxed/floxed mice that allowed temporal analysis of the impact of BRG1 inactivation in the embryo. The BRG1floxed/floxed/Cre-TM embryos exhibited edema and hemorrhage at embryonic day-13 and began to die. BRG1 deficient embryos had abnormal lymphatic sac linings with fewer LYVE1 positive lymphatic endothelial cells. Indeed, loss of BRG1 attenuated expression of a subset of lymphatic genes in-vivo. Furthermore, BRG1 binds at the promoters of COUP-TFII and LYVE1, suggesting that BRG1 modulates expression of these genes in the developing embryos. Conversely, re-expression of BRG1 in cells lacking endogenous BRG1 resulted in induction of lymphatic gene expression in-vitro, suggesting that BRG1 was both required and sufficient for lymphatic gene expression. These studies provide important insights into intrinsic regulation of BRG1-mediated lymphatic-gene expression, and further an understanding of lymphatic gene dysregulation in lymphedema and other disease conditions.
Keywords: BRG1; LYVE1; development; lymphatic; lymphedema.
Conflict of interest statement
CONFLICTS OF INTEREST None.
Figures
Similar articles
-
Brg1 Enables Rapid Growth of the Early Embryo by Suppressing Genes That Regulate Apoptosis and Cell Growth Arrest.Mol Cell Biol. 2016 Jul 14;36(15):1990-2010. doi: 10.1128/MCB.01101-15. Print 2016 Aug 1. Mol Cell Biol. 2016. PMID: 27185875 Free PMC article.
-
Temporally controlled targeted somatic mutagenesis in embryonic surface ectoderm and fetal epidermal keratinocytes unveils two distinct developmental functions of BRG1 in limb morphogenesis and skin barrier formation.Development. 2005 Oct;132(20):4533-44. doi: 10.1242/dev.02019. Development. 2005. PMID: 16192310
-
Loss of endothelial Tie1 receptor impairs lymphatic vessel development-brief report.Arterioscler Thromb Vasc Biol. 2010 Feb;30(2):207-9. doi: 10.1161/ATVBAHA.109.196618. Epub 2009 Nov 12. Arterioscler Thromb Vasc Biol. 2010. PMID: 19910638
-
Lymphatic endothelial cells, lymphedematous lymphangiogenesis, and molecular control of edema formation.Lymphat Res Biol. 2008;6(3-4):123-37. doi: 10.1089/lrb.2008.1005. Lymphat Res Biol. 2008. PMID: 19093784 Review.
-
Regulation of lymphatic vascular morphogenesis: Implications for pathological (tumor) lymphangiogenesis.Exp Cell Res. 2013 Jul 1;319(11):1618-25. doi: 10.1016/j.yexcr.2013.01.016. Epub 2013 Feb 6. Exp Cell Res. 2013. PMID: 23395992 Review.
Cited by
-
Low Efficacy of Genetic Tests for the Diagnosis of Primary Lymphedema Prompts Novel Insights into the Underlying Molecular Pathways.Int J Mol Sci. 2022 Jul 3;23(13):7414. doi: 10.3390/ijms23137414. Int J Mol Sci. 2022. PMID: 35806420 Free PMC article.
-
MiRNA-155 regulates lymphangiogenesis in natural killer/T-cell lymphoma by targeting BRG1.Cancer Biol Ther. 2019;20(1):31-41. doi: 10.1080/15384047.2018.1504721. Epub 2018 Oct 9. Cancer Biol Ther. 2019. PMID: 30299211 Free PMC article.
-
The core SWI/SNF catalytic subunit Brg1 regulates nephron progenitor cell proliferation and differentiation.Dev Biol. 2020 Aug 15;464(2):176-187. doi: 10.1016/j.ydbio.2020.05.008. Epub 2020 Jun 3. Dev Biol. 2020. PMID: 32504627 Free PMC article.
References
-
- Yang Y, Oliver G. Transcriptional Control of Lymphatic Endothelial Cell Type Specification. In: Kiefer F, Schulte-Merker S, editors. Developmental Aspects of the Lymphatic Vascular System. Springer Vienna; 2014. pp. 5–22.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

