Epidemiological and ecological determinants of Zika virus transmission in an urban setting
- PMID: 28887877
- PMCID: PMC5638629
- DOI: 10.7554/eLife.29820
Epidemiological and ecological determinants of Zika virus transmission in an urban setting
Abstract
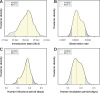
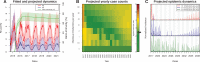
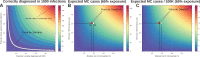
The Zika virus has emerged as a global public health concern. Its rapid geographic expansion is attributed to the success of Aedes mosquito vectors, but local epidemiological drivers are still poorly understood. Feira de Santana played a pivotal role in the Chikungunya epidemic in Brazil and was one of the first urban centres to report Zika infections. Using a climate-driven transmission model and notified Zika case data, we show that a low observation rate and high vectorial capacity translated into a significant attack rate during the 2015 outbreak, with a subsequent decline in 2016 and fade-out in 2017 due to herd-immunity. We find a potential Zika-related, low risk for microcephaly per pregnancy, but with significant public health impact given high attack rates. The balance between the loss of herd-immunity and viral re-importation will dictate future transmission potential of Zika in this urban setting.
Keywords: Zika; epidemiology; global health; herd-immunity; infectious disease; mathematical model; microbiology; virus.
Conflict of interest statement
No competing interests declared.
Figures



Similar articles
-
[Zika virus - ancient virus gets new life in a new ecosystem. Microcephaly and Guillain-Barre syndrome are possible consequences when there is no background herd immunity in the population].Lakartidningen. 2016 Mar 10;113:DX9X. Lakartidningen. 2016. PMID: 26978815 Swedish.
-
The Convergence of a Virus, Mosquitoes, and Human Travel in Globalizing the Zika Epidemic.J Community Health. 2016 Jun;41(3):674-9. doi: 10.1007/s10900-016-0177-7. J Community Health. 2016. PMID: 26969497 Review.
-
Zika virus: Endemic and epidemic ranges of Aedes mosquito transmission.J Infect Public Health. 2017 Jan-Feb;10(1):120-123. doi: 10.1016/j.jiph.2016.09.008. Epub 2016 Oct 1. J Infect Public Health. 2017. PMID: 27707632
-
Mapping global environmental suitability for Zika virus.Elife. 2016 Apr 19;5:e15272. doi: 10.7554/eLife.15272. Elife. 2016. PMID: 27090089 Free PMC article.
-
Zika virus outbreak: a review of neurological complications, diagnosis, and treatment options.J Neurovirol. 2018 Jun;24(3):255-272. doi: 10.1007/s13365-018-0614-8. Epub 2018 Feb 13. J Neurovirol. 2018. PMID: 29441490 Review.
Cited by
-
Impact of age-specific immunity on the timing and burden of the next Zika virus outbreak.PLoS Negl Trop Dis. 2019 Dec 26;13(12):e0007978. doi: 10.1371/journal.pntd.0007978. eCollection 2019 Dec. PLoS Negl Trop Dis. 2019. PMID: 31877200 Free PMC article.
-
Return of the founder Chikungunya virus to its place of introduction into Brazil is revealed by genomic characterization of exanthematic disease cases.Emerg Microbes Infect. 2019 Dec 27;9(1):53-57. doi: 10.1080/22221751.2019.1701954. eCollection 2020. Emerg Microbes Infect. 2019. PMID: 31880218 Free PMC article.
-
Impact of recent and future climate change on vector-borne diseases.Ann N Y Acad Sci. 2019 Jan;1436(1):157-173. doi: 10.1111/nyas.13950. Epub 2018 Aug 18. Ann N Y Acad Sci. 2019. PMID: 30120891 Free PMC article. Review.
-
Adverse outcomes of pregnancy-associated Zika virus infection.Semin Perinatol. 2018 Apr;42(3):155-167. doi: 10.1053/j.semperi.2018.02.003. Epub 2018 Mar 6. Semin Perinatol. 2018. PMID: 29523447 Free PMC article. Review.
-
Genomic Epidemiology Reconstructs the Introduction and Spread of Zika Virus in Central America and Mexico.Cell Host Microbe. 2018 Jun 13;23(6):855-864.e7. doi: 10.1016/j.chom.2018.04.017. Epub 2018 May 24. Cell Host Microbe. 2018. PMID: 29805095 Free PMC article.
References
-
- Bewick S, Fagan WF, Calabrese JM, Agusto F. Zika Virus: Endemic Versus Epidemic Dynamics and Implications for Disease Spread in the Americas. bioRxiv. 2016:041897
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

