Grass Carp Follisatin: Molecular Cloning, Functional Characterization, Dopamine D1 Regulation at Pituitary Level, and Implication in Growth Hormone Regulation
- PMID: 28883808
- PMCID: PMC5574371
- DOI: 10.3389/fendo.2017.00211
Grass Carp Follisatin: Molecular Cloning, Functional Characterization, Dopamine D1 Regulation at Pituitary Level, and Implication in Growth Hormone Regulation
Abstract
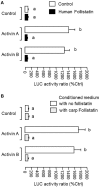
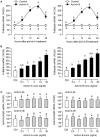
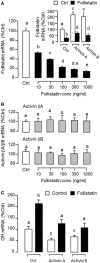
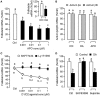
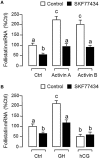
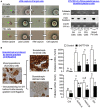
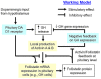
Activin is involved in pituitary hormone regulation and its pituitary actions can be nullified by local production of its binding protein follistatin. In our recent study with grass carp, local release of growth hormone (GH) was shown to induce activin expression at pituitary level, which in turn could exert an intrapituitary feedback to inhibit GH synthesis and secretion. To further examine the activin/follistatin system in the carp pituitary, grass carp follistatin was cloned and confirmed to be single-copy gene widely expressed at tissue level. At the pituitary level, follistatin signals could be located in carp somatotrophs, gonadotrophs, and lactotrophs. Functional expression also revealed that carp follistatin was effective in neutralizing activin's action in stimulating target promoter with activin-responsive elements. In grass carp pituitary cells, follistatin co-treatment was found to revert activin inhibition on GH mRNA expression. Meanwhile, follistatin mRNA levels could be up-regulated by local production of activin but the opposite was true for dopaminergic activation with dopamine (DA) or its agonist apomorphine. Since GH stimulation by DA via pituitary D1 receptor is well-documented in fish models, the receptor specificity for follistatin regulation by DA was also investigated. Using a pharmacological approach, the inhibitory effect of DA on follistatin gene expression was confirmed to be mediated by pituitary D1 but not D2 receptor. Furthermore, activation of D1 receptor by the D1-specific agonist SKF77434 was also effective in blocking follistatin mRNA expression induced by activin and GH treatment both in carp pituitary cells as well as in carp somatotrophs enriched by density gradient centrifugation. These results, as a whole, suggest that activin can interact with dopaminergic input from the hypothalamus to regulate follistatin expression in carp pituitary, which may contribute to GH regulation by activin/follistatin system via autocrine/paracrine mechanisms.
Keywords: activin; dopamine; follistatin; grass carp; growth hormone; pituitary.
Figures

Similar articles
-
Activin/follistatin system in grass carp pituitary cells: - Regulation by local release of growth hormone and luteinizing hormone and its functional role in growth hormone synthesis and secretion.PLoS One. 2017 Jun 29;12(6):e0179789. doi: 10.1371/journal.pone.0179789. eCollection 2017. PLoS One. 2017. PMID: 28662143 Free PMC article.
-
Somatostatin inhibits (d-Arg6, Pro9-NEt) salmon gonadotropin-releasing hormone- and dopamine D1-stimulated growth hormone release from perifused pituitary cells of chinese grass carp, ctenopharyngodon idellus.Gen Comp Endocrinol. 1998 Apr;110(1):29-45. doi: 10.1006/gcen.1997.7045. Gen Comp Endocrinol. 1998. PMID: 9514844
-
Regulation of dopamine D2 receptor expression in grass carp pituitary cells: a possible mechanism for dopaminergic modification of luteinizing hormone synthesis.Gen Comp Endocrinol. 2011 Aug 1;173(1):48-55. doi: 10.1016/j.ygcen.2011.04.024. Epub 2011 May 6. Gen Comp Endocrinol. 2011. PMID: 21570980
-
Feedback regulation of growth hormone synthesis and secretion in fish and the emerging concept of intrapituitary feedback loop.Comp Biochem Physiol A Mol Integr Physiol. 2006 Jul;144(3):284-305. doi: 10.1016/j.cbpa.2005.11.021. Epub 2006 Jan 9. Comp Biochem Physiol A Mol Integr Physiol. 2006. PMID: 16406825 Review.
-
Intrapituitary interactions: another level of endocrine regulation.Clin Exp Pharmacol Physiol. 2001 Mar;28(3):237-8. doi: 10.1046/j.1440-1681.2001.03420.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11236133 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

