Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial
- PMID: 28882536
- PMCID: PMC5622179
- DOI: 10.1016/S1470-2045(17)30622-8
Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial
Abstract
Background: For many years, first-line treatment for locally advanced or metastatic soft-tissue sarcoma has been doxorubicin. This study compared gemcitabine and docetaxel versus doxorubicin as first-line treatment for advanced or metastatic soft-tissue sarcoma.
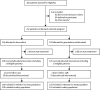
Methods: The GeDDiS trial was a randomised controlled phase 3 trial done in 24 UK hospitals and one Swiss Group for Clinical Cancer Research (SAKK) hospital. Eligible patients had histologically confirmed locally advanced or metastatic soft-tissue sarcoma of Trojani grade 2 or 3, disease progression before enrolment, and no previous chemotherapy for sarcoma or previous doxorubicin for any cancer. Patients were randomly assigned 1:1 to receive six cycles of intravenous doxorubicin 75 mg/m2 on day 1 every 3 weeks, or intravenous gemcitabine 675 mg/m2 on days 1 and 8 and intravenous docetaxel 75 mg/m2 on day 8 every 3 weeks. Treatment was assigned using a minimisation algorithm incorporating a random element. Randomisation was stratified by age (≤18 years vs >18 years) and histological subtype. The primary endpoint was the proportion of patients alive and progression free at 24 weeks in the intention-to-treat population. Adherence to treatment and toxicity were analysed in the safety population, consisting of all patients who received at least one dose of their randomised treatment. The trial was registered with the European Clinical Trials (EudraCT) database (no 2009-014907-29) and with the International Standard Randomised Controlled Trial registry (ISRCTN07742377), and is now closed to patient entry.
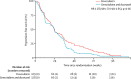
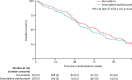
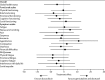
Findings: Between Dec 3, 2010, and Jan 20, 2014, 257 patients were enrolled and randomly assigned to the two treatment groups (129 to doxorubicin and 128 to gemcitabine and docetaxel). Median follow-up was 22 months (IQR 15·7-29·3). The proportion of patients alive and progression free at 24 weeks did not differ between those who received doxorubicin versus those who received gemcitabine and docetaxel (46·3% [95% CI 37·5-54·6] vs 46·4% [37·5-54·8]); median progression-free survival (23·3 weeks [95% CI 19·6-30·4] vs 23·7 weeks [18·1-20·0]; hazard ratio [HR] for progression-free survival 1·28, 95% CI 0·99-1·65, p=0·06). The most common grade 3 and 4 adverse events were neutropenia (32 [25%] of 128 patients who received doxorubicin and 25 [20%] of 126 patients who received gemcitabine and docetaxel), febrile neutropenia (26 [20%] and 15 [12%]), fatigue (eight [6%] and 17 [14%]), oral mucositis (18 [14%] and two [2%]), and pain (ten [8%] and 13 [10%]). The three most common serious adverse events, representing 111 (39%) of all 285 serious adverse events recorded, were febrile neutropenia (27 [17%] of 155 serious adverse events in patients who received doxorubicin and 15 [12%] of 130 serious adverse events in patients who received gemcitabine and docetaxel, fever (18 [12%] and 19 [15%]), and neutropenia (22 [14%] and ten [8%]). 154 (60%) of 257 patients died in the intention-to-treat population: 74 (57%) of 129 patients in the doxorubicin group and 80 (63%) of 128 in the gemcitabine and docetaxel group. No deaths were related to the treatment, but two deaths were due to a combination of disease progression and treatment.
Interpretation: Doxorubicin should remain the standard first-line treatment for most patients with advanced soft-tissue sarcoma. These results provide evidence for clinicians to consider with their patients when selecting first-line treatment for locally advanced or metastatic soft-tissue sarcoma.
Funding: Cancer Research UK, Sarcoma UK, and Clinical Trial Unit Kantonsspital St Gallen.
Copyright © 2017 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
GeDDiS: insight into frontline therapy in soft tissue sarcoma.Lancet Oncol. 2017 Oct;18(10):1297-1299. doi: 10.1016/S1470-2045(17)30672-1. Epub 2017 Sep 4. Lancet Oncol. 2017. PMID: 28882535 No abstract available.
Similar articles
-
Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial.Lancet Oncol. 2014 Apr;15(4):415-23. doi: 10.1016/S1470-2045(14)70063-4. Epub 2014 Mar 5. Lancet Oncol. 2014. PMID: 24618336 Clinical Trial.
-
Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial.Lancet Oncol. 2017 Jun;18(6):812-822. doi: 10.1016/S1470-2045(17)30334-0. Epub 2017 May 9. Lancet Oncol. 2017. PMID: 28499583 Clinical Trial.
-
Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial.Lancet Oncol. 2017 Aug;18(8):1089-1103. doi: 10.1016/S1470-2045(17)30381-9. Epub 2017 Jun 23. Lancet Oncol. 2017. PMID: 28651927 Free PMC article. Clinical Trial.
-
Update on gemcitabine and docetaxel combination therapy for primary and metastatic sarcomas.Curr Opin Oncol. 2010 Jul;22(4):356-61. doi: 10.1097/CCO.0b013e32833aafef. Curr Opin Oncol. 2010. PMID: 20520541 Review.
-
[Chemotherapy options for patients with advanced soft-tissue sarcoma beyond anthracyclines].Bull Cancer. 2010 Jun;97(6):679-86. doi: 10.1684/bdc.2010.1119. Bull Cancer. 2010. PMID: 20483708 Review. French.
Cited by
-
Beneficial Use of the Combination of Gemcitabine and Dacarbazine in Advanced Soft Tissue Sarcomas: Real-World Data.Cancers (Basel). 2024 Jan 8;16(2):267. doi: 10.3390/cancers16020267. Cancers (Basel). 2024. PMID: 38254758 Free PMC article.
-
Prognostic Role of OX40, LAG-3, TIM-3 and PD-L1 Expression in Bone and Soft Tissue Sarcomas.J Clin Med. 2024 Jun 20;13(12):3620. doi: 10.3390/jcm13123620. J Clin Med. 2024. PMID: 38930150 Free PMC article.
-
Unmet Medical Needs and Future Perspectives for Leiomyosarcoma Patients-A Position Paper from the National LeioMyoSarcoma Foundation (NLMSF) and Sarcoma Patients EuroNet (SPAEN).Cancers (Basel). 2021 Feb 20;13(4):886. doi: 10.3390/cancers13040886. Cancers (Basel). 2021. PMID: 33672607 Free PMC article. Review.
-
Sarcoma of the Uterus. Guideline of the DGGG, OEGGG and SGGG (S2k-Level, AWMF Registry No. 015/074, April 2021).Geburtshilfe Frauenheilkd. 2022 Dec 1;82(12):1337-1367. doi: 10.1055/a-1897-5124. eCollection 2022 Dec. Geburtshilfe Frauenheilkd. 2022. PMID: 36467974 Free PMC article.
-
Antibiotic therapy augments the efficacy of gemcitabine-containing regimens for advanced cancer: a retrospective study.Cancer Manag Res. 2019 Aug 22;11:7953-7965. doi: 10.2147/CMAR.S215697. eCollection 2019. Cancer Manag Res. 2019. PMID: 31686910 Free PMC article.
References
-
- Francis MDN, Charman J, Lawrence G, Grimer R. Bone and soft tissue sarcomas UK Incidence and Survival: 1996 to 2010. National Cancer Intelligence Network. 2013. http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_s... (accessed Aug 25, 2017).
-
- Judson I, Verweij J, Gelderblom H. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. - PubMed
-
- Karavasilis V, Seddon BM, Ashley S, Al-Muderis O, Fisher C, Judson I. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112:1585–1591. - PubMed
-
- Ryan CW, Merimsky O, Agulnik M. PICASSO III: a phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016;34:3898–3905. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

