Chaperone-mediated autophagy substrate proteins in cancer
- PMID: 28881704
- PMCID: PMC5584305
- DOI: 10.18632/oncotarget.17583
Chaperone-mediated autophagy substrate proteins in cancer
Abstract
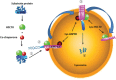
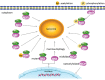
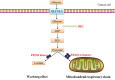
All intracellular proteins undergo continuous synthesis and degradation. Chaperone-mediated autophagy (CMA) is necessary to maintain cellular homeostasis through turnover of cytosolic proteins (substrate proteins). This degradation involves a series of substrate proteins including both cancer promoters and suppressors. Since activating or inhibiting CMA pathway to treat cancer is still debated, targeting to the CMA substrate proteins provides a novel direction. We summarize the cancer-associated substrate proteins which are degraded by CMA. Consequently, CMA substrate proteins catalyze the glycolysis which contributes to the Warburg effect in cancer cells. The fact that the degradation of substrate proteins based on the CMA can be altered by posttranslational modifications such as phosphorylation or acetylation. In conclusion, targeting to CMA substrate proteins develops into a new anticancer therapeutic approach.
Keywords: Warburg effect; cancer; chaperone-mediated autophagy; glycolysis; substrate proteins.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have declared no potential conflicts of interest.
Figures
Similar articles
-
Chaperone-Mediated Autophagy.Adv Exp Med Biol. 2019;1206:435-452. doi: 10.1007/978-981-15-0602-4_20. Adv Exp Med Biol. 2019. PMID: 31776997 Review.
-
Acetylation of translationally controlled tumor protein promotes its degradation through chaperone-mediated autophagy.Eur J Cell Biol. 2017 Mar;96(2):83-98. doi: 10.1016/j.ejcb.2016.12.002. Epub 2017 Jan 17. Eur J Cell Biol. 2017. PMID: 28110910
-
Chaperone-mediated autophagy: roles in neurodegeneration.Transl Neurodegener. 2014 Sep 21;3:20. doi: 10.1186/2047-9158-3-20. eCollection 2014. Transl Neurodegener. 2014. PMID: 25276349 Free PMC article. Review.
-
Chaperone-Mediated Autophagy and Its Implications for Neurodegeneration and Cancer.Int J Gen Med. 2022 Jun 15;15:5635-5649. doi: 10.2147/IJGM.S368364. eCollection 2022. Int J Gen Med. 2022. PMID: 35734200 Free PMC article. Review.
-
Chaperone mediated autophagy to the rescue: A new-fangled target for the treatment of neurodegenerative diseases.Mol Cell Neurosci. 2015 May;66(Pt A):29-36. doi: 10.1016/j.mcn.2015.01.003. Epub 2015 Feb 25. Mol Cell Neurosci. 2015. PMID: 25724482 Review.
Cited by
-
Verteporfin disrupts multiple steps of autophagy and regulates p53 to sensitize osteosarcoma cells.Cancer Cell Int. 2021 Jan 14;21(1):52. doi: 10.1186/s12935-020-01720-y. Cancer Cell Int. 2021. PMID: 33446200 Free PMC article.
-
Clusterin overexpression in mice exacerbates diabetic phenotypes but suppresses tumor progression in a mouse melanoma model.Aging (Albany NY). 2021 Mar 10;13(5):6485-6505. doi: 10.18632/aging.202788. Epub 2021 Mar 10. Aging (Albany NY). 2021. PMID: 33744871 Free PMC article.
-
ARD1 contributes to IKKβ-mediated breast cancer tumorigenesis.Cell Death Dis. 2018 Aug 28;9(9):860. doi: 10.1038/s41419-018-0921-2. Cell Death Dis. 2018. PMID: 30154412 Free PMC article.
-
The Multifaceted Roles of Autophagy in Infectious, Obstructive, and Malignant Airway Diseases.Biomedicines. 2022 Aug 11;10(8):1944. doi: 10.3390/biomedicines10081944. Biomedicines. 2022. PMID: 36009490 Free PMC article. Review.
-
Targetome analysis of chaperone-mediated autophagy in cancer cells.Autophagy. 2019 Sep;15(9):1558-1571. doi: 10.1080/15548627.2019.1586255. Epub 2019 Mar 20. Autophagy. 2019. PMID: 30821613 Free PMC article.
References
-
- Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12(Suppl 2):1535–41. - PubMed
-
- Du P, Cao H, Wu HR, Zhu BS, Wang HW, Gu CW, Xing CG, Chen W. Blocking Bcl-2 leads to autophagy activation and cell death of the HEPG2 liver cancer cell line. Asian Pac J Cancer Prev. 2013;14:5849–54. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

