Use of a neonatal rat system as a bioincubator to generate adult-like mature cardiomyocytes from human and mouse pluripotent stem cells
- PMID: 28880277
- PMCID: PMC5600470
- DOI: 10.1038/nprot.2017.089
Use of a neonatal rat system as a bioincubator to generate adult-like mature cardiomyocytes from human and mouse pluripotent stem cells
Abstract
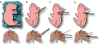
Pluripotent stem cells (PSCs), including induced PSCs, hold great potential for personalized disease modeling, drug testing and cell-based therapeutics. However, cells differentiated from PSCs remain immature in a dish, and thus there are serious caveats to their use in modeling adult-onset diseases such as cardiomyopathies and Alzheimer's disease. By taking advantage of knowledge gained about mammalian development and from bioinformatics analyses, we recently developed a neonatal rat system that enables maturation of PSC-derived cardiomyocytes into cardiomyocytes analogous to those seen in adult animals. Here we describe a detailed protocol that describes how to initiate the in vitro differentiation of mouse and human PSCs into cardiac progenitor cells, followed by intramyocardial delivery of the progenitor cells into neonatal rat hearts, in vivo incubation and analysis. The entire process takes ∼6 weeks, and the resulting cardiomyocytes can be analyzed for morphology, function and gene expression. The neonatal system provides a valuable tool for understanding the maturation and pathogenesis of adult human heart muscle cells, and this concept may be expanded to maturing other PSC-derived cell types, including those containing mutations that lead to the development of diseases in the adult.
Conflict of interest statement
Authors declare no competing financial interests.
Figures

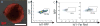


Similar articles
-
Neonatal Transplantation Confers Maturation of PSC-Derived Cardiomyocytes Conducive to Modeling Cardiomyopathy.Cell Rep. 2017 Jan 10;18(2):571-582. doi: 10.1016/j.celrep.2016.12.040. Cell Rep. 2017. PMID: 28076798 Free PMC article.
-
Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues.Adv Drug Deliv Rev. 2016 Jan 15;96:110-34. doi: 10.1016/j.addr.2015.04.019. Epub 2015 May 5. Adv Drug Deliv Rev. 2016. PMID: 25956564 Free PMC article. Review.
-
MicroRNA-mediated maturation of human pluripotent stem cell-derived cardiomyocytes: Towards a better model for cardiotoxicity?Food Chem Toxicol. 2016 Dec;98(Pt A):17-24. doi: 10.1016/j.fct.2016.05.025. Epub 2016 Jun 3. Food Chem Toxicol. 2016. PMID: 27265266 Review.
-
A Simple Protocol for the Generation of Cardiomyocytes from Human Pluripotent Stem Cells.Methods Mol Biol. 2016;1307:379-83. doi: 10.1007/7651_2013_54. Methods Mol Biol. 2016. PMID: 24297314
-
Engineering human ventricular heart muscles based on a highly efficient system for purification of human pluripotent stem cell-derived ventricular cardiomyocytes.Stem Cell Res Ther. 2017 Sep 29;8(1):202. doi: 10.1186/s13287-017-0651-x. Stem Cell Res Ther. 2017. PMID: 28962583 Free PMC article.
Cited by
-
Establishing normal metabolism and differentiation in hepatocellular carcinoma cells by culturing in adult human serum.Sci Rep. 2018 Aug 3;8(1):11685. doi: 10.1038/s41598-018-29763-2. Sci Rep. 2018. PMID: 30076349 Free PMC article.
-
Harnessing cell pluripotency for cardiovascular regenerative medicine.Nat Biomed Eng. 2018 Jun;2(6):392-398. doi: 10.1038/s41551-018-0244-8. Epub 2018 Jun 11. Nat Biomed Eng. 2018. PMID: 31011193 Free PMC article. Review.
-
Advances in Stem Cell Modeling of Dystrophin-Associated Disease: Implications for the Wider World of Dilated Cardiomyopathy.Front Physiol. 2020 May 12;11:368. doi: 10.3389/fphys.2020.00368. eCollection 2020. Front Physiol. 2020. PMID: 32477154 Free PMC article. Review.
-
In Vitro Generation of Heart Field-specific Cardiac Progenitor Cells.J Vis Exp. 2019 Jul 3;(149):10.3791/59826. doi: 10.3791/59826. J Vis Exp. 2019. PMID: 31329174 Free PMC article.
-
Maturing heart muscle cells: Mechanisms and transcriptomic insights.Semin Cell Dev Biol. 2021 Nov;119:49-60. doi: 10.1016/j.semcdb.2021.04.019. Epub 2021 May 2. Semin Cell Dev Biol. 2021. PMID: 33952430 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

