Direct integrin binding to insulin-like growth factor-2 through the C-domain is required for insulin-like growth factor receptor type 1 (IGF1R) signaling
- PMID: 28873464
- PMCID: PMC5584928
- DOI: 10.1371/journal.pone.0184285
Direct integrin binding to insulin-like growth factor-2 through the C-domain is required for insulin-like growth factor receptor type 1 (IGF1R) signaling
Abstract
We have reported that integrins crosstalk with growth factors through direct binding to growth factors (e.g., fibroblast growth factor-1, insulin-like growth factor 1 (IGF1), neuregulin-1, fractalkine) and subsequent ternary complex formation with cognate receptor [e.g., integrin/IGF1/IGF1 receptor (IGF1R)]. IGF1 and IGF2 are overexpressed in cancer and major therapeutic targets. We previously reported that IGF1 binds to integrins ανβ3 and α6β4, and the R36E/R37E mutant in the C-domain of IGF1 is defective integrin binding and signaling functions of IGF1, and acts as an antagonist of IGF1R. We studied if integrins play a role in the signaling functions of IGF2, another member of the IGF family. Here we describe that IGF2 specifically binds to integrins ανβ3 and α6β4, and induced proliferation of CHO cells (IGF1R+) that express ανβ3 or α6β4 (β3- or α6β4-CHO cells). Arg residues to Glu at positions 24, 34, 37 and/or 38 in or close to the C-domain of IGF2 play a critical role in binding to integrins and signaling functions. The R24E/R37E/R38E, R34E/R37E/R38E, and R24E/R34E/R37E/R38E mutants were defective in integrin binding and IGF2 signaling. These mutants suppressed proliferation induced by WT IGF2, suggesting that they are dominant-negative antagonists of IGF1R. These results suggest that IGF2 also requires integrin binding for signaling functions, and the IGF2 mutants that cannot bind to integrins act as antagonists of IGF1R. The present study defines the role of the C-domain in integrin binding and signaling.
Conflict of interest statement
Figures
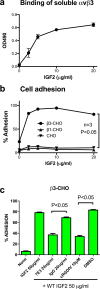
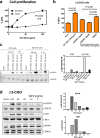
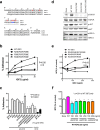
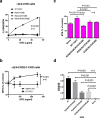
Similar articles
-
Cross-talk between integrin α6β4 and insulin-like growth factor-1 receptor (IGF1R) through direct α6β4 binding to IGF1 and subsequent α6β4-IGF1-IGF1R ternary complex formation in anchorage-independent conditions.J Biol Chem. 2012 Apr 6;287(15):12491-500. doi: 10.1074/jbc.M111.304170. Epub 2012 Feb 20. J Biol Chem. 2012. PMID: 22351760 Free PMC article.
-
An integrin binding-defective mutant of insulin-like growth factor-1 (R36E/R37E IGF1) acts as a dominant-negative antagonist of the IGF1 receptor (IGF1R) and suppresses tumorigenesis but still binds to IGF1R.J Biol Chem. 2013 Jul 5;288(27):19593-603. doi: 10.1074/jbc.M113.470872. Epub 2013 May 21. J Biol Chem. 2013. PMID: 23696648 Free PMC article.
-
Insulin-like growth factor (IGF) signaling requires αvβ3-IGF1-IGF type 1 receptor (IGF1R) ternary complex formation in anchorage independence, and the complex formation does not require IGF1R and Src activation.J Biol Chem. 2013 Feb 1;288(5):3059-69. doi: 10.1074/jbc.M112.412536. Epub 2012 Dec 14. J Biol Chem. 2013. PMID: 23243309 Free PMC article.
-
Crosstalk between insulin-like growth factor (IGF) receptor and integrins through direct integrin binding to IGF1.Cytokine Growth Factor Rev. 2017 Apr;34:67-72. doi: 10.1016/j.cytogfr.2017.01.003. Epub 2017 Feb 3. Cytokine Growth Factor Rev. 2017. PMID: 28190785 Free PMC article. Review.
-
Synstatin: a selective inhibitor of the syndecan-1-coupled IGF1R-αvβ3 integrin complex in tumorigenesis and angiogenesis.FEBS J. 2013 May;280(10):2207-15. doi: 10.1111/febs.12160. Epub 2013 Feb 24. FEBS J. 2013. PMID: 23375101 Free PMC article. Review.
Cited by
-
Prenatal diagnosis of Silver-Russell syndrome with 8q12 deletion including the PLAG1 gene: a case report and review.Front Genet. 2024 May 17;15:1387649. doi: 10.3389/fgene.2024.1387649. eCollection 2024. Front Genet. 2024. PMID: 38826801 Free PMC article.
-
Drugging IGF-1R in cancer: New insights and emerging opportunities.Genes Dis. 2022 Mar 23;10(1):199-211. doi: 10.1016/j.gendis.2022.03.002. eCollection 2023 Jan. Genes Dis. 2022. PMID: 37013053 Free PMC article. Review.
-
FGF1 Suppresses Allosteric Activation of β3 Integrins by FGF2: A Potential Mechanism of Anti-Inflammatory and Anti-Thrombotic Action of FGF1.Biomolecules. 2024 Jul 23;14(8):888. doi: 10.3390/biom14080888. Biomolecules. 2024. PMID: 39199276 Free PMC article.
-
Preclinical Study of Human Bone Marrow-Derived Mesenchymal Stem Cells Using a 3-Dimensional Manufacturing Setting for Enhancing Spinal Fusion.Stem Cells Transl Med. 2022 Oct 21;11(10):1072-1088. doi: 10.1093/stcltm/szac052. Stem Cells Transl Med. 2022. PMID: 36180050 Free PMC article.
-
Nidogen-1/NID1 Function and Regulation during Progression and Metastasis of Colorectal Cancer.Cancers (Basel). 2023 Nov 7;15(22):5316. doi: 10.3390/cancers15225316. Cancers (Basel). 2023. PMID: 38001576 Free PMC article.
References
-
- Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215 PubMed doi: 10.1186/gb-2007-8-5-215 . - DOI - PMC - PubMed
-
- Marelli UK, Rechenmacher F, Sobahi TR, Mas-Moruno C, Kessler H. Tumor Targeting via Integrin Ligands. Front Oncol. 2013;3:222 doi: 10.3389/fonc.2013.00222 ; PubMed Central PMCID: PMCPMC3757457. - DOI - PMC - PubMed
-
- Mori S, Wu CY, Yamaji S, Saegusa J, Shi B, Ma Z, et al. Direct Binding of Integrin {alpha}v{beta}3 to FGF1 Plays a Role in FGF1 Signaling. J Biol Chem. 2008;283(26):18066–75. Epub 2008/04/29. doi: 10.1074/jbc.M801213200 . - DOI - PMC - PubMed
-
- Fujita M, Takada YK, Takada Y. Insulin-like Growth Factor (IGF) Signaling Requires alphavbeta3-IGF1-IGF Type 1 Receptor (IGF1R) Ternary Complex Formation in Anchorage Independence, and the Complex Formation Does Not Require IGF1R and Src Activation. J Biol Chem. 2013;288(5):3059–69. Epub 2012/12/18. doi: 10.1074/jbc.M112.412536 ; PubMed Central PMCID: PMC3561530. - DOI - PMC - PubMed
-
- Saegusa J, Yamaji S, Ieguchi K, Wu CY, Lam KS, Liu FT, et al. The direct binding of insulin-like growth factor-1 (IGF-1) to integrin alphavbeta3 is involved in IGF-1 signaling. J Biol Chem. 2009;284(36):24106–14. Epub 2009/07/07. doi: 10.1074/jbc.M109.013201 . - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

