DT-13 Ameliorates TNF-α-Induced Vascular Endothelial Hyperpermeability via Non-Muscle Myosin IIA and the Src/PI3K/Akt Signaling Pathway
- PMID: 28855900
- PMCID: PMC5557769
- DOI: 10.3389/fimmu.2017.00925
DT-13 Ameliorates TNF-α-Induced Vascular Endothelial Hyperpermeability via Non-Muscle Myosin IIA and the Src/PI3K/Akt Signaling Pathway
Abstract
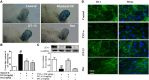
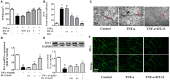
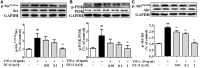
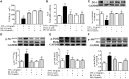
DT-13(25(R,S)-ruscogenin-1-O-[β-d-glucopyranosyl-(1→2)][β-d-xylopyranosyl-(1→3)]-β-d-fucopyranoside) has been identified as an important factor in TNF-α-induced vascular inflammation. However, the effect of DT-13 on TNF-α-induced endothelial permeability and the potential molecular mechanisms remain unclear. Hence, this study was undertaken to elucidate the protective effect of DT-13 on TNF-α-induced endothelial permeability and the underlying mechanisms in vivo and in vitro. The in vivo results showed that DT-13 could ameliorate endothelial permeability in mustard oil-induced plasma leakage in the skin and modulate ZO-1 organization. In addition, the in vitro results showed that pretreatment with DT-13 could increase the transendothelial electrical resistance value and decrease the sodium fluorescein permeability coefficient. Moreover, DT-13 altered the mRNA and protein levels of ZO-1 as determined by real-time PCR, Western blotting, and immunofluorescence analyses. DT-13 treatment decreased the phosphorylations of Src, PI3K, and Akt in TNF-α-treated human umbilical vein endothelial cells (HUVECs). Further analyses with PP2 (10 µM, inhibitor of Src) indicated that DT-13 modulated endothelial permeability in TNF-α-induced HUVECs in an Src-dependent manner. LY294002 (10 µM, PI3K inhibitor) also had the same effect on DT-13 but did not affect phosphorylation of Src. Following decreased expression of non-muscle myosin IIA (NMIIA), the effect of DT-13 on the phosphorylations of Src, PI3K, and Akt was abolished. This study provides pharmacological evidence showing that DT-13 significantly ameliorated the TNF-α-induced vascular endothelial hyperpermeability through modulation of the Src/PI3K/Akt pathway and NMIIA, which play an important role in this process.
Keywords: DT-13; Src/PI3K/Akt; non-muscle myosin IIA; tight junctions; vascular endothelial hyperpermeability.
Figures

Similar articles
-
The saponin DT-13 attenuates tumor necrosis factor-α-induced vascular inflammation associated with Src/NF-кB/MAPK pathway modulation.Int J Biol Sci. 2015 Jun 11;11(8):970-81. doi: 10.7150/ijbs.11635. eCollection 2015. Int J Biol Sci. 2015. PMID: 26157351 Free PMC article.
-
DT-13 ameliorates TNF-α-induced nitric oxide production in the endothelium in vivo and in vitro.Biochem Biophys Res Commun. 2018 Jan 1;495(1):1175-1181. doi: 10.1016/j.bbrc.2017.11.009. Epub 2017 Nov 21. Biochem Biophys Res Commun. 2018. PMID: 29162452
-
Anti-inflammatory mechanism of ulinastatin: Inhibiting the hyperpermeability of vascular endothelial cells induced by TNF-α via the RhoA/ROCK signal pathway.Int Immunopharmacol. 2017 May;46:220-227. doi: 10.1016/j.intimp.2017.03.007. Epub 2017 Mar 19. Int Immunopharmacol. 2017. PMID: 28329735
-
c-Src-dependent transactivation of PDGFR contributes to TNF-α-induced MMP-9 expression and functional impairment in osteoblasts.Bone. 2014 Mar;60:186-97. doi: 10.1016/j.bone.2013.12.014. Epub 2013 Dec 18. Bone. 2014. PMID: 24361597
-
c-Src-dependent MAPKs/AP-1 activation is involved in TNF-α-induced matrix metalloproteinase-9 expression in rat heart-derived H9c2 cells.Biochem Pharmacol. 2013 Apr 15;85(8):1115-23. doi: 10.1016/j.bcp.2013.01.013. Epub 2013 Jan 24. Biochem Pharmacol. 2013. PMID: 23353699
Cited by
-
Distinguishing host responses, extensive viral dissemination and long-term viral RNA persistence in domestic sheep experimentally infected with Crimean-Congo haemorrhagic fever virus Kosovo Hoti.Emerg Microbes Infect. 2024 Dec;13(1):2302103. doi: 10.1080/22221751.2024.2302103. Epub 2024 Jan 22. Emerg Microbes Infect. 2024. PMID: 38189080 Free PMC article.
-
Ruscogenin attenuated tight junction injury and tumor migration in colorectal liver metastasis mice via regulating TRAP1.Transl Cancer Res. 2021 Mar;10(3):1470-1483. doi: 10.21037/tcr-20-2968. Transl Cancer Res. 2021. PMID: 35116472 Free PMC article.
-
Ophiopogon Saponin C1 Inhibits Lung Tumors by Stabilizing Endothelium Permeability via Inhibition of PKCδ.Int J Biol Sci. 2020 Jan 1;16(3):396-407. doi: 10.7150/ijbs.34978. eCollection 2020. Int J Biol Sci. 2020. PMID: 32015677 Free PMC article.
-
In vitro analysis of VEGF-mediated endothelial permeability and the potential therapeutic role of Anti-VEGF in severe dengue.Biochem Biophys Rep. 2024 Aug 22;39:101814. doi: 10.1016/j.bbrep.2024.101814. eCollection 2024 Sep. Biochem Biophys Rep. 2024. PMID: 39263317 Free PMC article.
-
Fumonisin B1 Inhibits Cell Proliferation and Decreases Barrier Function of Swine Umbilical Vein Endothelial Cells.Toxins (Basel). 2021 Dec 3;13(12):863. doi: 10.3390/toxins13120863. Toxins (Basel). 2021. PMID: 34941701 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

