Amyloid β-42 induces neuronal apoptosis by targeting mitochondria
- PMID: 28849115
- PMCID: PMC5647099
- DOI: 10.3892/mmr.2017.7203
Amyloid β-42 induces neuronal apoptosis by targeting mitochondria
Abstract
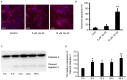
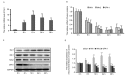
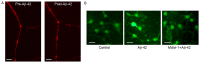
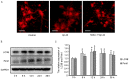
Alzheimer's disease (AD), with a typical pathological hallmark of amyloid‑beta (Aβ)‑containing plaques and neurofibrillary tangles, is one of the most common types of chronic neurodegenerative diseases. Aβ oligomers serve a crucial role in the pathogenesis of AD, and lead to neuronal loss. However, the precise mechanism of Aβ oligomers in AD remains to be elucidated. The present study demonstrated that 10 µM Aβ‑42 activated the caspase signaling pathway, and induced significant apoptosis in primary cultured mouse cerebral cortical neurons. The results of reverse transcription‑quantitative polymerase chain reaction and western blotting demonstrated that Aβ‑42 (10 µM) also significantly upregulated the transcription and expression of the mitochondrial fission protein dynamin‑related protein 1 (Drp1), and downregulated the transcription and expression of mitochondrial fusion proteins, including mitofusin 1/2 (Mfn1/2) and mitochondrial dynamin like GTPase (OPA‑1). Neurons were transfected with pDsRed2‑Mito for mitochondrial imaging, which revealed that 10 µM Aβ‑42 induced mitochondrial fission in cortical neurons. In addition, 2',7'‑dichlorodihydrofluorescein diacetate and tetramethylrhodamine ethyl ester staining indicated that Aβ‑42 increased the reactive oxygen species (ROS) level and reduced mitochondrial membrane potential in neurons. Inhibition of Drp1 activity by Mdivi‑1 efficiently prevented Aβ‑42‑induced ROS production and disruption of mitochondrial membrane potential. Loss of mitochondrial membrane potential may activate PTEN‑induced putative kinase 1 (Pink1), the prominent sensor for mitochondrial damage, and trigger the process of mitophagy to remove the damaged mitochondria. In the present study, western blotting revealed that the levels of autophagy marker microtubule‑associated proteins 1A/1B light chain 3B (LC3B) and Pink1 were upregulated after Aβ‑42 stimulation. In conclusion, these data indicated that Aβ‑42 induces neuronal apoptosis by targeting mitochondria, including promotion of mitochondrial fission, disruption of mitochondrial membrane potential, increasing intracellular ROS level and activation of the process of mitophagy. Therefore, mitochondria may represent a potential therapeutic target for AD in the future.
Figures

Similar articles
-
Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis.Biochim Biophys Acta. 2016 Nov;1863(11):2820-2834. doi: 10.1016/j.bbamcr.2016.09.003. Epub 2016 Sep 4. Biochim Biophys Acta. 2016. PMID: 27599716
-
Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy.Autophagy. 2019 Dec;15(12):2107-2125. doi: 10.1080/15548627.2019.1596494. Epub 2019 Mar 28. Autophagy. 2019. PMID: 30894073 Free PMC article.
-
Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease.Cells. 2019 May 22;8(5):488. doi: 10.3390/cells8050488. Cells. 2019. PMID: 31121890 Free PMC article. Review.
-
Ethanol-activated CaMKII signaling induces neuronal apoptosis through Drp1-mediated excessive mitochondrial fission and JNK1-dependent NLRP3 inflammasome activation.Cell Commun Signal. 2020 Aug 12;18(1):123. doi: 10.1186/s12964-020-00572-3. Cell Commun Signal. 2020. PMID: 32787872 Free PMC article.
-
Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Mitochondrial Dysfunction for Alzheimer's Disease.Mol Neurobiol. 2022 Jan;59(1):535-555. doi: 10.1007/s12035-021-02612-6. Epub 2021 Nov 2. Mol Neurobiol. 2022. PMID: 34725778 Review.
Cited by
-
The role of amyloid β in the pathological mechanism of GNE myopathy.Neurol Sci. 2022 Nov;43(11):6309-6321. doi: 10.1007/s10072-022-06301-7. Epub 2022 Jul 29. Neurol Sci. 2022. PMID: 35904705 Free PMC article. Review.
-
Protective effect of andrographolide against STZ induced Alzheimer's disease in experimental rats: possible neuromodulation and Aβ(1-42) analysis.Inflammopharmacology. 2021 Aug;29(4):1157-1168. doi: 10.1007/s10787-021-00843-6. Epub 2021 Jul 7. Inflammopharmacology. 2021. PMID: 34235591
-
Nicotinamide, a Poly [ADP-Ribose] Polymerase 1 (PARP-1) Inhibitor, as an Adjunctive Therapy for the Treatment of Alzheimer's Disease.Front Aging Neurosci. 2020 Aug 13;12:255. doi: 10.3389/fnagi.2020.00255. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32903806 Free PMC article. Review.
-
Memory-Enhancing Effects of Origanum majorana Essential Oil in an Alzheimer's Amyloid beta1-42 Rat Model: A Molecular and Behavioral Study.Antioxidants (Basel). 2020 Sep 26;9(10):919. doi: 10.3390/antiox9100919. Antioxidants (Basel). 2020. PMID: 32993114 Free PMC article.
-
Nicotinamide phosphoribosyltransferase‑related signaling pathway in early Alzheimer's disease mouse models.Mol Med Rep. 2019 Dec;20(6):5163-5171. doi: 10.3892/mmr.2019.10782. Epub 2019 Oct 30. Mol Med Rep. 2019. PMID: 31702813 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

