LC3-Associated Phagocytosis and Inflammation
- PMID: 28847720
- PMCID: PMC5743439
- DOI: 10.1016/j.jmb.2017.08.012
LC3-Associated Phagocytosis and Inflammation
Abstract
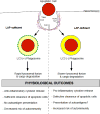
LC3-associated phagocytosis (LAP) is a novel form of non-canonical autophagy where LC3 (microtubule-associated protein 1A/1B-light chain 3) is conjugated to phagosome membranes using a portion of the canonical autophagy machinery. The impact of LAP to immune regulation is best characterized in professional phagocytes, in particular macrophages, where LAP has instrumental roles in the clearance of extracellular particles including apoptotic cells and pathogens. Binding of dead cells via receptors present on the macrophage surface results in the translocation of the autophagy machinery to the phagosome and ultimately LC3 conjugation. These events promote a rapid form of phagocytosis that produces an "immunologically silent" clearance of the apoptotic cells. Consequences of LAP deficiency include a decreased capacity to clear dying cells and the establishment of a lupus-like autoimmune disease in mice. The ability of LAP to attenuate autoimmunity likely occurs through the dampening of pro-inflammatory signals upon engulfment of dying cells and prevention of autoantigen presentation to other immune cells. However, it remains unclear how LAP shapes both the activation and outcome of the immune response at the molecular level. Herein, we provide a detailed review of LAP and its known roles in the immune response and provide further speculation on the putative mechanisms by which LAP may regulate immune function, perhaps through the metabolic reprogramming and polarization of macrophages.
Keywords: LC3-associated phagocytosis; autophagy; efferocytosis; immune regulation; metabolism.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
The clearance of dying cells: table for two.Cell Death Differ. 2016 Jun;23(6):915-26. doi: 10.1038/cdd.2015.172. Epub 2016 Mar 18. Cell Death Differ. 2016. PMID: 26990661 Free PMC article. Review.
-
LC3-associated phagocytosis at a glance.J Cell Sci. 2019 Feb 20;132(5):jcs222984. doi: 10.1242/jcs.222984. J Cell Sci. 2019. PMID: 30787029 Free PMC article. Review.
-
NLRX1 Facilitates Histoplasma capsulatum-Induced LC3-Associated Phagocytosis for Cytokine Production in Macrophages.Front Immunol. 2018 Dec 3;9:2761. doi: 10.3389/fimmu.2018.02761. eCollection 2018. Front Immunol. 2018. PMID: 30559741 Free PMC article.
-
LC3-Associated Phagocytosis in Myeloid Cells Promotes Tumor Immune Tolerance.Cell. 2018 Oct 4;175(2):429-441.e16. doi: 10.1016/j.cell.2018.08.061. Epub 2018 Sep 20. Cell. 2018. PMID: 30245008 Free PMC article.
-
Enhancement of LC3-associated efferocytosis for the alleviation of intestinal inflammation.Autophagy. 2024 Jun;20(6):1442-1443. doi: 10.1080/15548627.2024.2311548. Epub 2024 Feb 15. Autophagy. 2024. PMID: 38311819
Cited by
-
LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury.Nat Cell Biol. 2020 Oct;22(10):1252-1263. doi: 10.1038/s41556-020-00583-9. Epub 2020 Sep 28. Nat Cell Biol. 2020. PMID: 32989250
-
LC3-Associated Phagocytosis (LAP): A Potentially Influential Mediator of Efferocytosis-Related Tumor Progression and Aggressiveness.Front Oncol. 2020 Aug 5;10:1298. doi: 10.3389/fonc.2020.01298. eCollection 2020. Front Oncol. 2020. PMID: 32850405 Free PMC article. Review.
-
Thioredoxin-Interacting Protein Promotes Phagosomal Acidification Upon Exposure to Escherichia coli Through Inflammasome-Mediated Caspase-1 Activation in Macrophages.Front Immunol. 2019 Nov 12;10:2636. doi: 10.3389/fimmu.2019.02636. eCollection 2019. Front Immunol. 2019. PMID: 31781121 Free PMC article.
-
IL-27 inhibits anti- Mycobacterium tuberculosis innate immune activity of primary human macrophages.Tuberculosis (Edinb). 2023 Mar;139:102326. doi: 10.1016/j.tube.2023.102326. Epub 2023 Feb 24. Tuberculosis (Edinb). 2023. PMID: 36863206 Free PMC article.
-
Mechanisms and Pathophysiological Roles of the ATG8 Conjugation Machinery.Cells. 2019 Aug 25;8(9):973. doi: 10.3390/cells8090973. Cells. 2019. PMID: 31450711 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

