Deep Sequencing Details the Cross-over Map of Chimeric Genes in Two Porcine Reproductive and Respiratory Syndrome Virus Infectious Clones
- PMID: 28839504
- PMCID: PMC5543688
- DOI: 10.2174/1874357901711010049
Deep Sequencing Details the Cross-over Map of Chimeric Genes in Two Porcine Reproductive and Respiratory Syndrome Virus Infectious Clones
Abstract
Background: Recombination is an important contributor to the genetic diversity of most viruses. A reverse genetics system using green fluorescence protein (GFP)- and enhanced GFP (EGFP)-expressing infectious clones was developed to study the requirements for recombination. However, it is still unclear what types of cross-over events occurred to produce the viable offspring.
Objective: We utilized 454 sequencing to infer recombination events in this system.
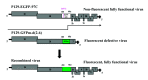
Method: Two porcine reproductive and respiratory syndrome virus (PRRSV) infectious clones, P129-EGFP-97C and P129-GFPm-d (2-6), were co-transfected into HEK-293T cells. P129-EGFP-97C is a fully functional virus that contains a non-fluorescent EGFP. P129-GFPm-d (2-6) is a defective virus but contains a fluorescent GFPm. Successful recombination was evident by the appearance of fully functional progeny virus that expresses fluorescence. Total RNA was extracted from infected cells expressing fluorescence, and the entire fluorescent gene was amplified to prepare an amplicon library for 454 sequencing.
Results: Deep sequencing showed that the nucleotide identities changed from ~37% (in the variable region from 21nt to 165nt) to 20% (T289C) to ~38% (456-651nt) then to 100% (672-696nt) when compared to EGFP. The results indicated that cross-over events occurred in three conserved regions (166-288nt, 290-455nt, 652-671nt), which were also supported by sequence alignments. Remarkably, the short conserved region (652-671nt) showed to be a cross-over hotspot. In addition, four cross-over patterns (two single and two double cross-over) might be used to produce viable recombinants.
Conclusion: The reverse genetics system incorporating the use of high throughput sequencing creates a genetic platform to study the generation of viable recombinant viruses.
Keywords: Cross-over map; Deep sequencing; Green fluorescent protein; Infectious clone; Porcine reproductive and respiratory syndrome virus (PRRSV); Viable recombinant.
Figures

Similar articles
-
Porcine reproductive and respiratory syndrome virus as a vector: immunogenicity of green fluorescent protein and porcine circovirus type 2 capsid expressed from dedicated subgenomic RNAs.Virology. 2009 Jun 20;389(1-2):91-9. doi: 10.1016/j.virol.2009.03.036. Epub 2009 May 6. Virology. 2009. PMID: 19423145
-
An Expeditious Neutralization Assay for Porcine Reproductive and Respiratory Syndrome Virus Based on a Recombinant Virus Expressing Green Fluorescent Protein.Curr Issues Mol Biol. 2024 Jan 23;46(2):1047-1063. doi: 10.3390/cimb46020066. Curr Issues Mol Biol. 2024. PMID: 38392184 Free PMC article.
-
Expression and stability of foreign tags inserted into nsp2 of porcine reproductive and respiratory syndrome virus (PRRSV).Virus Res. 2007 Sep;128(1-2):106-14. doi: 10.1016/j.virusres.2007.04.019. Epub 2007 Jun 5. Virus Res. 2007. PMID: 17553585
-
Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction.Vaccine. 2015 Aug 7;33(33):4069-80. doi: 10.1016/j.vaccine.2015.06.092. Epub 2015 Jul 4. Vaccine. 2015. PMID: 26148878 Review.
-
Engineering the PRRS virus genome: updates and perspectives.Vet Microbiol. 2014 Dec 5;174(3-4):279-295. doi: 10.1016/j.vetmic.2014.10.007. Epub 2014 Oct 23. Vet Microbiol. 2014. PMID: 25458419 Free PMC article. Review.
References
-
- Lunney J.K., Fang Y., Ladinig A., Chen N., Li Y., Rowland R.R., Renukaradhya G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016;4:129–154. - PubMed
-
- Galetto R., Giacomoni V., Veron M., Negroni M. Dissection of a circumscribed recombination hot spot in HIV-1 after a single infectious cycle. J. Biol. Chem. 2006;281:2711–2720. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
