Cephalotaxus Alkaloids
- PMID: 28838429
- PMCID: PMC7110560
- DOI: 10.1016/bs.alkal.2017.07.001
Cephalotaxus Alkaloids
Abstract
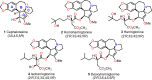
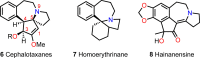
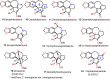
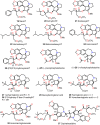
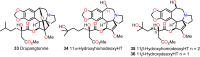
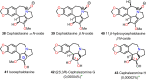
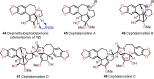
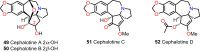
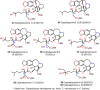
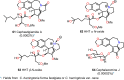
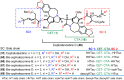
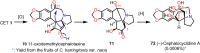
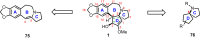
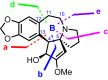
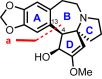
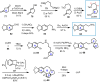
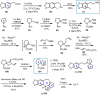
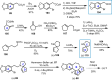
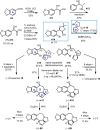
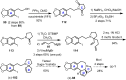
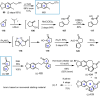
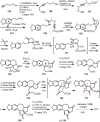
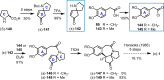
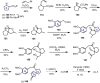
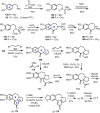
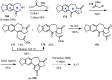
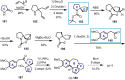
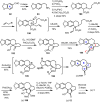
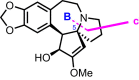
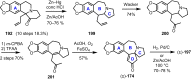
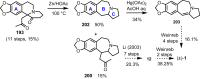
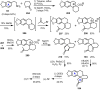
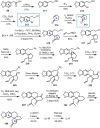
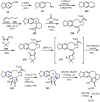
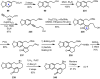
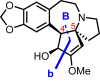
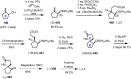
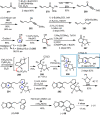
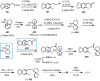
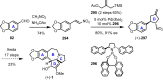
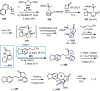
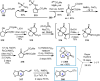
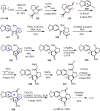
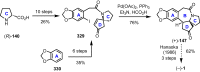
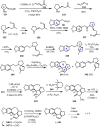
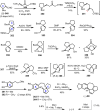
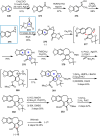
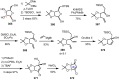
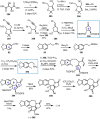
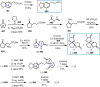
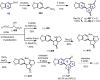
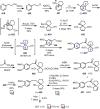
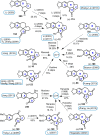
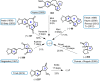
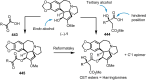
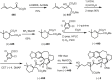
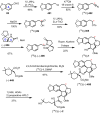
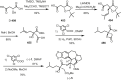
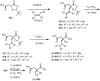
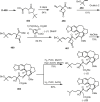
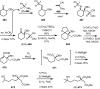
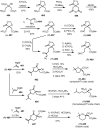
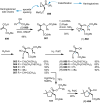
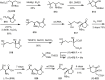
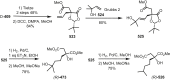
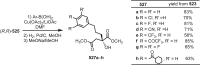
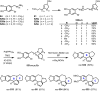
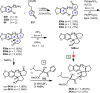
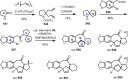
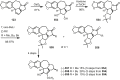
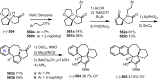
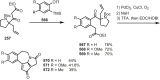
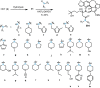
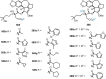
Cephalotaxus alkaloids represent a family of plant secondary metabolites known for 60 years. Significant activity against leukemia in mice was demonstrated for extracts of Cephalotaxus. Cephalotaxine (CET) (1), the major alkaloid of this series was isolated from Cephalotaxus drupacea species by Paudler in 1963. The subsequent discovery of promising antitumor activity among new Cephalotaxus derivatives reported by Chinese, Japanese, and American teams triggered extensive structure elucidation and biological studies in this family. The structural feature of this cephalotaxane family relies mainly on its tetracyclic alkaloid backbone, which comprises an azaspiranic 1-azaspiro[4.4]nonane unit (rings C and D) and a benzazepine ring system (rings A and B), which is linked by its C3 alcohol function to a chiral oxygenated side chain by a carboxylic function alpha to a tetrasubstituted carbon center. The botanical distribution of these alkaloids is limited to the Cephalotaxus genus (Cephalotaxaceae). The scope of biological activities of the Cephalotaxus alkaloids is mainly centered on the antileukemic activity of homoharringtonine (HHT) (2), which in particular demonstrated marked benefits in the treatment of orphan myeloid leukemia and was approved as soon as 2009 by European Medicine Agency and by US Food and Drug Administration in 2012. Its exact mechanism of action was partly elucidated and it was early recognized that HHT (2) inhibited protein synthesis at the level of the ribosome machinery. Interestingly, after a latency period of two decades, the topic of Cephalotaxus alkaloids reemerged as a prolific source of new natural structures. To date, more than 70 compounds have been identified and characterized. Synthetic studies also regained attention during the past two decades, and numerous methodologies were developed to access the first semisynthetic HHT (2) of high purity suitable for clinical studies, and then high grade enantiomerically pure CET (1), HHT (2), and analogs.
Keywords: Alkaloid; Antiparasitic agent; Antiviral; Cancer; Cephalotaxine; Cephalotaxus; Harringtonine; Homoharringtonine; Leukemia; Protein synthesis inhibitor.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
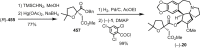
Similar articles
-
[Studies on the alkaloids of Cephalotaxus. VII. Structures and semi-synthesis of two anticancer cephalotaxine esters].Yao Xue Xue Bao. 1992;27(3):173-7. Yao Xue Xue Bao. 1992. PMID: 1414381 Chinese.
-
Anti-varicella-zoster virus activity of cephalotaxine esters in vitro.J Microbiol. 2019 Jan;57(1):74-79. doi: 10.1007/s12275-019-8514-z. Epub 2018 Nov 19. J Microbiol. 2019. PMID: 30456755 Free PMC article.
-
Cephalotaxine-type alkaloids from the seeds of Cephalotaxus fortunei and their cytotoxic activities.Phytochemistry. 2021 Nov;191:112903. doi: 10.1016/j.phytochem.2021.112903. Epub 2021 Aug 9. Phytochemistry. 2021. PMID: 34384922
-
The Securinega alkaloids.Alkaloids Chem Biol. 2015;74:1-120. doi: 10.1016/bs.alkal.2014.11.001. Alkaloids Chem Biol. 2015. PMID: 25845059 Review.
-
The genus Cephalotaxus: source of homoharringtonine and related anticancer alkaloids.Cancer Treat Rep. 1976 Aug;60(8):1157-70. Cancer Treat Rep. 1976. PMID: 791485 Review.
Cited by
-
Chelerythrine Chloride: A Potential Rumen Microbial Urease Inhibitor Screened by Targeting UreG.Int J Mol Sci. 2021 Jul 30;22(15):8212. doi: 10.3390/ijms22158212. Int J Mol Sci. 2021. PMID: 34360977 Free PMC article.
-
Cephalotaxine inhibits Zika infection by impeding viral replication and stability.Biochem Biophys Res Commun. 2020 Feb 19;522(4):1052-1058. doi: 10.1016/j.bbrc.2019.12.012. Epub 2019 Dec 7. Biochem Biophys Res Commun. 2020. PMID: 31818462 Free PMC article.
-
Synthesis of 1-Azaspiro[4.4]nonane Derivatives Enabled by Domino Radical Bicyclization Involving Formation and Capture of Alkoxyaminyl Radicals.ACS Omega. 2019 Dec 4;4(25):21100-21114. doi: 10.1021/acsomega.9b02515. eCollection 2019 Dec 17. ACS Omega. 2019. PMID: 31867503 Free PMC article.
-
Constructing an artificial short route for cell-free biosynthesis of the phenethylisoquinoline scaffold.Synth Syst Biotechnol. 2023 Sep 22;8(4):610-617. doi: 10.1016/j.synbio.2023.09.003. eCollection 2023 Dec. Synth Syst Biotechnol. 2023. PMID: 37781172 Free PMC article.
-
Phytochemicals as Chemo-Preventive Agents and Signaling Molecule Modulators: Current Role in Cancer Therapeutics and Inflammation.Int J Mol Sci. 2022 Dec 12;23(24):15765. doi: 10.3390/ijms232415765. Int J Mol Sci. 2022. PMID: 36555406 Free PMC article. Review.
References
-
- Huang L., Xue Z. In: Manske R.H.F., editor. Vol. 23. Academic Press; New York: 1984. pp. 157–226. (The Alkaloids).
-
- Miah M.A.J., Hudlicky T., Reed J.W. In: Cordell G.A., editor. Vol. 51. Academic Press; San Diego: 1998. pp. 199–269. (The Alkaloids).
-
- Mei W., Wu J., Dai H. Zhongcaoyao. 2006;37:452–458.
-
- Itokawa H., Wang X., Lee K.-H. In: Anticancer Agents from Natural Products. Cragg G.M., Kingston D.G.I., Newman D., editors. Brunner-Routledge Psychology Press, Taylor & Francis Group; Boca Raton, Florida: 2005. pp. 47–70.
-
- Itokawa H., Hitotsuyanagi Y., Lee K.-H. In: Anticancer Agents from Natural Products, 2nd ed. Cragg G.M., Kingston D.G.I., Newman D.J., editors. CRC Press/Taylor & Francis Group; Boca Raton, Florida: 2012. pp. 65–93.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

