Immunogenicity and efficacy of DNA/MVA HIV vaccines in rhesus macaque models
- PMID: 28838267
- PMCID: PMC6120759
- DOI: 10.1080/14760584.2017.1371594
Immunogenicity and efficacy of DNA/MVA HIV vaccines in rhesus macaque models
Abstract
Despite 30 years of research on HIV, a vaccine to prevent infection and limit disease progression remains elusive. The RV144 trial showed moderate, but significant protection in humans and highlighted the contribution of antibody responses directed against HIV envelope as an important immune correlate for protection. Efforts to further build upon the progress include the use of a heterologous prime-boost regimen using DNA as the priming agent and the attenuated vaccinia virus, Modified Vaccinia Ankara (MVA), as a boosting vector for generating protective HIV-specific immunity. Areas covered: In this review, we summarize the immunogenicity of DNA/MVA vaccines in non-human primate models and describe the efficacy seen in SIV infection models. We discuss immunological correlates of protection determined by these studies and potential approaches for improving the protective immunity. Additionally, we describe the current progress of DNA/MVA vaccines in human trials. Expert commentary: Efforts over the past decade have provided the opportunity to better understand the dynamics of vaccine-induced immune responses and immune correlates of protection against HIV. Based on what we have learned, we outline multiple areas where the field will likely focus on in the next five years.
Keywords: AIDS; DNA; HIV vaccine; MVA; T cell responses; antibody responses; heterologous; modified vaccinia ankara; prime-boost.
Figures
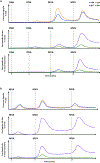

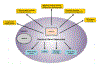
Similar articles
-
Virus-Like Particles Displaying Trimeric Simian Immunodeficiency Virus (SIV) Envelope gp160 Enhance the Breadth of DNA/Modified Vaccinia Virus Ankara SIV Vaccine-Induced Antibody Responses in Rhesus Macaques.J Virol. 2016 Sep 12;90(19):8842-54. doi: 10.1128/JVI.01163-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466414 Free PMC article.
-
HIV-1 gp120 and Modified Vaccinia Virus Ankara (MVA) gp140 Boost Immunogens Increase Immunogenicity of a DNA/MVA HIV-1 Vaccine.J Virol. 2017 Nov 30;91(24):e01077-17. doi: 10.1128/JVI.01077-17. Print 2017 Dec 15. J Virol. 2017. PMID: 29021394 Free PMC article.
-
CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge.J Virol. 2014 Sep 1;88(17):9579-89. doi: 10.1128/JVI.00975-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920805 Free PMC article.
-
Drug evaluation: DNA/MVA prime-boost HIV vaccine.Curr Opin Investig Drugs. 2007 Feb;8(2):159-67. Curr Opin Investig Drugs. 2007. PMID: 17328232 Review.
-
Development of a DNA-MVA/HIVA vaccine for Kenya.Vaccine. 2002 May 6;20(15):1995-8. doi: 10.1016/s0264-410x(02)00085-3. Vaccine. 2002. PMID: 11983261 Review.
Cited by
-
HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge.Nat Commun. 2019 Feb 18;10(1):798. doi: 10.1038/s41467-019-08739-4. Nat Commun. 2019. PMID: 30778066 Free PMC article.
-
Intradermal but not intramuscular modified vaccinia Ankara immunizations protect against intravaginal tier2 simian-human immunodeficiency virus challenges in female macaques.Nat Commun. 2023 Aug 8;14(1):4789. doi: 10.1038/s41467-023-40430-7. Nat Commun. 2023. PMID: 37553348 Free PMC article.
-
Oral Coadministration of an Intramuscular DNA/Modified Vaccinia Ankara Vaccine for Simian Immunodeficiency Virus Is Associated with Better Control of Infection in Orally Exposed Infant Macaques.AIDS Res Hum Retroviruses. 2019 Mar;35(3):310-325. doi: 10.1089/AID.2018.0180. Epub 2018 Nov 27. AIDS Res Hum Retroviruses. 2019. PMID: 30303405 Free PMC article.
-
Features of Effective T Cell-Inducing Vaccines against Chronic Viral Infections.Front Immunol. 2018 Feb 16;9:276. doi: 10.3389/fimmu.2018.00276. eCollection 2018. Front Immunol. 2018. PMID: 29503649 Free PMC article. Review.
-
DNA Vaccine-Induced Long-Lasting Cytotoxic T Cells Targeting Conserved Elements of Human Immunodeficiency Virus Gag Are Boosted Upon DNA or Recombinant Modified Vaccinia Ankara Vaccination.Hum Gene Ther. 2018 Sep;29(9):1029-1043. doi: 10.1089/hum.2018.065. Epub 2018 Jun 21. Hum Gene Ther. 2018. PMID: 29869530 Free PMC article.
References
-
- Hemelaar J The origin and diversity of the HIV-1 pandemic. Trends in molecular medicine, 18(3), 182–192 (2012). - PubMed
-
- Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, Detours V. Evolutionary and immunological implications of contemporary HIV-1 variation. British medical bulletin, 58, 19–42 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
