IRAK-M alters the polarity of macrophages to facilitate the survival of Mycobacterium tuberculosis
- PMID: 28835201
- PMCID: PMC5569470
- DOI: 10.1186/s12866-017-1095-2
IRAK-M alters the polarity of macrophages to facilitate the survival of Mycobacterium tuberculosis
Abstract
Background: Intracellular bacterium, Mycobacterium tuberculosis (M. tb), infects specifically macrophages as host cells. IRAK-M, a member of IRAK family, is a negative regulator in TLR signaling and specifically expresses in monocytes and macrophages. The role of IRAK-M in intracellular growth of M. tb and macrophage polarization was explored, for deeply understanding the pathogenesis of M. tb, the significance of IRAK-M to innate immunity and pathogen-host interaction.
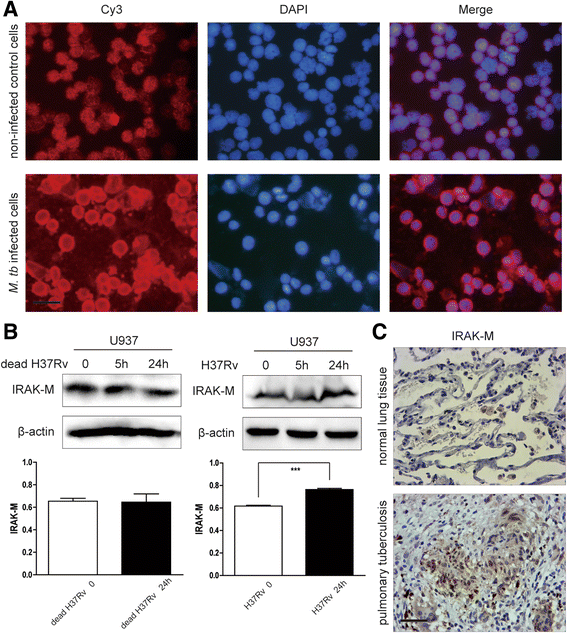
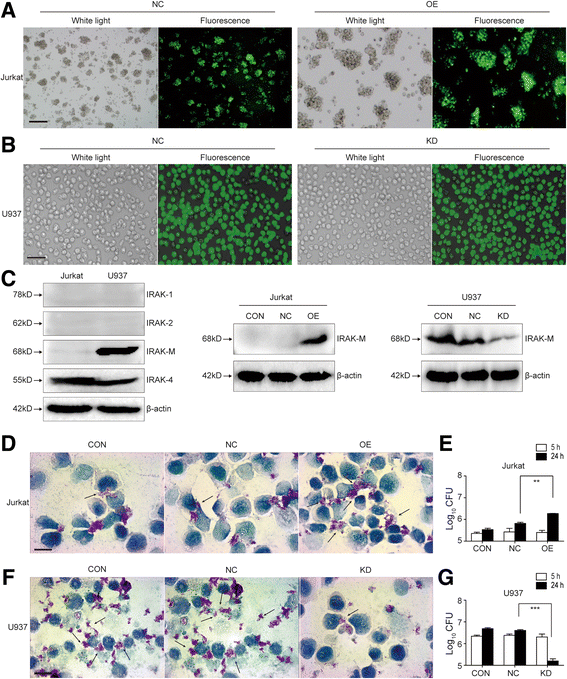
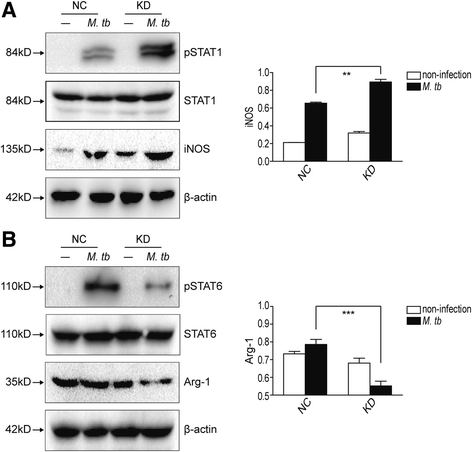
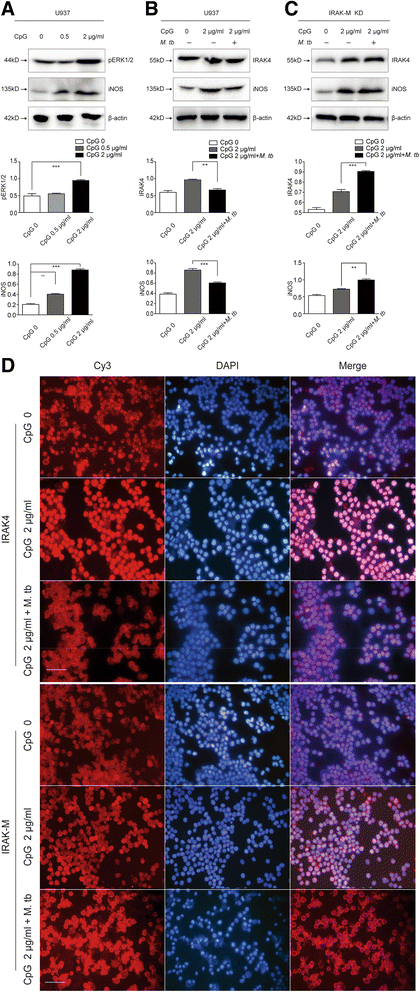
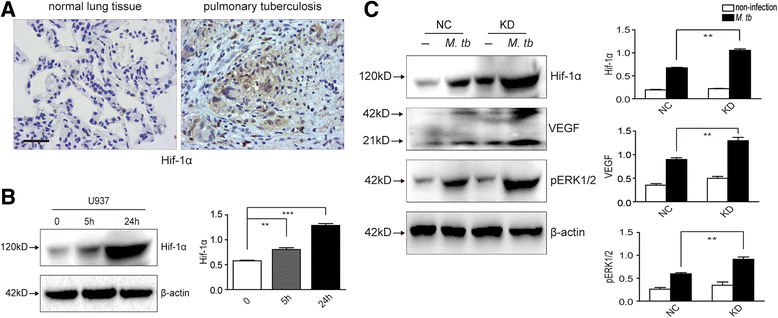
Methods: IRAK-M expression was detected in M. tb infected macrophages and in human lung tissue of pulmonary tuberculosis with immunofluorescence staining, Western blot and immunohistochemistry. IRAK-M knock-down and over-expressing cell strains were constructed and intracellular survival of M. tb was investigated by acid-fast staining and colony forming units. Molecular markers of M1-type (pSTAT1 and iNOS) and M2-type (pSTAT6 and Arg-1) macrophages were detected using Western blot in IRAK-M knockdown U937 cells infected with M. tb H37Rv. U937 cells were stimulated with immunostimulant CpG7909 into M1 status and then infected with M. tb H37Rv. Expression of IRAK-M, IRAK-4 and iNOS was detected with immunofluorescence staining and Western blot, to evaluate the effect of IRAK-M to CpG directed M1-type polarization of macrophages during M. tb infection. Molecules related with macrophage's bactericidal ability such as Hif-1 and phosphorylated ERK1/2 were detected with immunohistochemistry and Western blot.
Results: IRAK-M increased in M. tb infected macrophage cells and also in human lung tissue of pulmonary tuberculosis. IRAK-M over-expression resulted in higher bacterial load, while IRAK-M interference resulted in lower bacterial load in M. tb infected cells. During M. tb infection, IRAK-M knockdown induced M1-type, while inhibited M2-type polarization of macrophage. M1-type polarization of U937 cells induced by CpG7909 was inhibited by M. tb infection, which was reversed by IRAK-M knockdown in U937 cells. IRAK-M affected Hif-1 and MAPK signaling cascade during M. tb infection.
Conclusions: Conclusively, IRAK-M might alter the polarity of macrophages, to facilitate intracellular survival of M. tb and affect Th1-type immunity of the host, which is helpful to understanding the pathogenesis of M. tb.
Keywords: IRAK-M; Intracellular survival; Macrophage; Mycobacterium tuberculosis; Polarization.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest related to the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Lack of IL-1 Receptor-Associated Kinase-4 Leads to Defective Th1 Cell Responses and Renders Mice Susceptible to Mycobacterial Infection.J Immunol. 2016 Sep 1;197(5):1852-63. doi: 10.4049/jimmunol.1502157. Epub 2016 Jul 20. J Immunol. 2016. PMID: 27439514
-
GRN Activates TNFR2 to Promote Macrophage M2 Polarization Aggravating Mycobacterium Tuberculosis Infection.Front Biosci (Landmark Ed). 2024 Sep 24;29(9):332. doi: 10.31083/j.fbl2909332. Front Biosci (Landmark Ed). 2024. PMID: 39344332
-
Polarization of Human Monocyte-Derived Cells With Vitamin D Promotes Control of Mycobacterium tuberculosis Infection.Front Immunol. 2020 Jan 22;10:3157. doi: 10.3389/fimmu.2019.03157. eCollection 2019. Front Immunol. 2020. PMID: 32038652 Free PMC article.
-
Macrophage heterogeneity and plasticity in tuberculosis.J Leukoc Biol. 2019 Aug;106(2):275-282. doi: 10.1002/JLB.MR0318-095RR. Epub 2019 Apr 2. J Leukoc Biol. 2019. PMID: 30938876 Review.
-
Macrophage immunoregulatory pathways in tuberculosis.Semin Immunol. 2014 Dec;26(6):471-85. doi: 10.1016/j.smim.2014.09.010. Epub 2014 Oct 30. Semin Immunol. 2014. PMID: 25453226 Free PMC article. Review.
Cited by
-
Hacking the host: exploitation of macrophage polarization by intracellular bacterial pathogens.Pathog Dis. 2020 Feb 1;78(1):ftaa009. doi: 10.1093/femspd/ftaa009. Pathog Dis. 2020. PMID: 32068828 Free PMC article. Review.
-
Mutations in the Vicinity of the IRAK3 Guanylate Cyclase Center Impact Its Subcellular Localization and Ability to Modulate Inflammatory Signaling in Immortalized Cell Lines.Int J Mol Sci. 2023 May 10;24(10):8572. doi: 10.3390/ijms24108572. Int J Mol Sci. 2023. PMID: 37239919 Free PMC article.
-
HIV and the tuberculosis "set point": how HIV impairs alveolar macrophage responses to tuberculosis and sets the stage for progressive disease.Retrovirology. 2020 Sep 23;17(1):32. doi: 10.1186/s12977-020-00540-2. Retrovirology. 2020. PMID: 32967690 Free PMC article. Review.
-
Arginase 1 is involved in lacrimal hyposecretion in male NOD mice, a model of Sjögren's syndrome, regardless of dacryoadenitis status.J Physiol. 2020 Nov;598(21):4907-4925. doi: 10.1113/JP280090. Epub 2020 Aug 29. J Physiol. 2020. PMID: 32780506 Free PMC article.
-
Mycobacterial Trehalose 6,6'-Dimycolate-Induced M1-Type Inflammation.Am J Pathol. 2020 Feb;190(2):286-294. doi: 10.1016/j.ajpath.2019.10.006. Epub 2019 Nov 14. Am J Pathol. 2020. PMID: 31734231 Free PMC article.
References
-
- Jennewein J, Matuszak J, Walter S, Felmy B, Gendera K, Schatz V, Nowottny M, Liebsch G, Hensel M, Hardt WD, et al. Low-oxygen tensions found in Salmonella-infected gut tissue boost Salmonella replication in macrophages by impairing antimicrobial activity and augmenting Salmonella virulence. Cell Microbiol. 2015;17(12):1833–1847. doi: 10.1111/cmi.12476. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

