Three-Dimensional Analysis of Chloroplast Structures Associated with Virus Infection
- PMID: 28821590
- PMCID: PMC5761806
- DOI: 10.1104/pp.17.00871
Three-Dimensional Analysis of Chloroplast Structures Associated with Virus Infection
Abstract
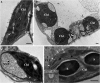
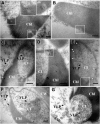
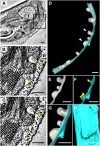
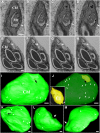
Chloroplasts are multifunctional organelles whose morphology is affected by environmental stresses. Although the three-dimensional (3D) architecture of thylakoid membranes has been reported previously, a 3D visualization of chloroplast under stress has not been explored. In this work, we used a positive-strand RNA ((+)RNA) virus, barley stripe mosaic virus (BSMV) to observe chloroplast structural changes during infection by electron tomography. The analyses revealed remodeling of the chloroplast membranes, characterized by the clustering of outer membrane-invaginated spherules in inner membrane-derived packets. Diverse morphologies of cytoplasmic invaginations (CIs) were evident with spherules at the periphery and different sized openings connecting the CIs to the cytoplasm. Immunoelectron microscopy of these viral components verified that the aberrant membrane structures were sites for BSMV replication. The BSMV αa replication protein localized at the surface of the chloroplasts and played a prominent role in eliciting chloroplast membrane rearrangements. In sum, our results have revealed the 3D structure of the chloroplasts induced by BSMV infection. These findings contribute to our understanding of chloroplast morphological changes under stress conditions and during assembly of plant (+)RNA virus replication complexes.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures

Similar articles
-
Three-dimensional reconstruction and comparison of vacuolar membranes in response to viral infection.J Integr Plant Biol. 2021 Feb;63(2):353-364. doi: 10.1111/jipb.13027. J Integr Plant Biol. 2021. PMID: 33085164
-
Morphogenesis of Endoplasmic Reticulum Membrane-Invaginated Vesicles during Beet Black Scorch Virus Infection: Role of Auxiliary Replication Protein and New Implications of Three-Dimensional Architecture.J Virol. 2015 Jun;89(12):6184-95. doi: 10.1128/JVI.00401-15. Epub 2015 Apr 1. J Virol. 2015. PMID: 25833056 Free PMC article.
-
Barley stripe mosaic virus-encoded proteins triple-gene block 2 and gammab localize to chloroplasts in virus-infected monocot and dicot plants, revealing hitherto-unknown roles in virus replication.J Gen Virol. 2006 Aug;87(Pt 8):2403-2411. doi: 10.1099/vir.0.81975-0. J Gen Virol. 2006. PMID: 16847137
-
The altered photosynthetic machinery during compatible virus infection.Curr Opin Virol. 2016 Apr;17:19-24. doi: 10.1016/j.coviro.2015.11.002. Epub 2015 Nov 30. Curr Opin Virol. 2016. PMID: 26651024 Review.
-
Wrapping membranes around plant virus infection.Curr Opin Virol. 2011 Nov;1(5):388-95. doi: 10.1016/j.coviro.2011.09.009. Epub 2011 Oct 14. Curr Opin Virol. 2011. PMID: 22440840 Review.
Cited by
-
Membrane Trafficking Proteins: A New Target to Identify Resistance to Viruses in Plants.Plants (Basel). 2021 Oct 9;10(10):2139. doi: 10.3390/plants10102139. Plants (Basel). 2021. PMID: 34685948 Free PMC article. Review.
-
Fusion of Mitochondria to 3-D Networks, Autophagy and Increased Organelle Contacts are Important Subcellular Hallmarks during Cold Stress in Plants.Int J Mol Sci. 2020 Nov 19;21(22):8753. doi: 10.3390/ijms21228753. Int J Mol Sci. 2020. PMID: 33228190 Free PMC article.
-
The Role of the Chloroplast in the Replication of Positive-Sense Single-Stranded Plant RNA Viruses.Front Plant Sci. 2018 Nov 27;9:1776. doi: 10.3389/fpls.2018.01776. eCollection 2018. Front Plant Sci. 2018. PMID: 30542365 Free PMC article.
-
A cytorhabdovirus phosphoprotein forms mobile inclusions trafficked on the actin/ER network for viral RNA synthesis.J Exp Bot. 2019 Aug 7;70(15):4049-4062. doi: 10.1093/jxb/erz195. J Exp Bot. 2019. PMID: 31020313 Free PMC article.
-
A sword or a buffet: plant endomembrane system in viral infections.Front Plant Sci. 2023 Aug 11;14:1226498. doi: 10.3389/fpls.2023.1226498. eCollection 2023. Front Plant Sci. 2023. PMID: 37636115 Free PMC article. Review.
References
-
- Allen TC. (1972) Subcellular responses of mesophyll cells to Wild cucumber mosaic virus. Virology 47: 467–474 - PubMed
-
- Ballantine JEM, Forde BJ (1970) The effect of light intensity and temperature on plant growth and chloroplast ultrastructure in soybean. Am J Bot 57: 1150–1159
-
- Baumeister W. (2002) Electron tomography: Towards visualizing the molecular organization of the cytoplasm. Curr Opin Struct Biol 12: 679–684 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

