Molecular processes involved in B cell acute lymphoblastic leukaemia
- PMID: 28819864
- PMCID: PMC5765206
- DOI: 10.1007/s00018-017-2620-z
Molecular processes involved in B cell acute lymphoblastic leukaemia
Abstract
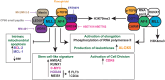
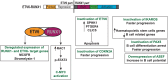
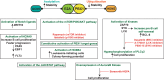
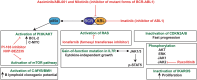
B cell leukaemia is one of the most frequent malignancies in the paediatric population, but also affects a significant proportion of adults in developed countries. The majority of infant and paediatric cases initiate the process of leukaemogenesis during foetal development (in utero) through the formation of a chromosomal translocation or the acquisition/deletion of genetic material (hyperdiploidy or hypodiploidy, respectively). This first genetic insult is the major determinant for the prognosis and therapeutic outcome of patients. B cell leukaemia in adults displays similar molecular features as its paediatric counterpart. However, since this disease is highly represented in the infant and paediatric population, this review will focus on this demographic group and summarise the biological, clinical and epidemiological knowledge on B cell acute lymphoblastic leukaemia of four well characterised subtypes: t(4;11) MLL-AF4, t(12;21) ETV6-RUNX1, t(1;19) E2A-PBX1 and t(9;22) BCR-ABL1.
Keywords: B cell acute lymphoblastic leukaemia; BCR-ABL1; E2A-PBX1; ETV6-RUNX1; MLL-AF4; Paediatric leukaemia.
Figures
Similar articles
-
Clinical characteristics and prognostic analysis of CDKN2A/2B gene in pediatric acute lymphoblastic leukemia: a retrospective case-control study.Hematology. 2025 Dec;30(1):2439606. doi: 10.1080/16078454.2024.2439606. Epub 2024 Dec 15. Hematology. 2025. PMID: 39676312
-
Pharmacogenetics of 6-mercaptopurine in a black Zimbabwean cohort treated for acute lymphoblastic leukaemia.Pharmacogenomics. 2023 Jun;24(8):449-457. doi: 10.2217/pgs-2023-0026. Epub 2023 May 30. Pharmacogenomics. 2023. PMID: 37248698 Free PMC article.
-
Paediatric Acute Lymphoblastic Leukaemia: A Narrative Review of Current Knowledge and Advancements.Curr Oncol Rep. 2024 Dec;26(12):1586-1599. doi: 10.1007/s11912-024-01608-4. Epub 2024 Nov 6. Curr Oncol Rep. 2024. PMID: 39503990 Review.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
The evolution of nutritional care in children and young people with acute lymphoblastic leukaemia: a narrative review.J Hum Nutr Diet. 2025 Feb;38(1):e13273. doi: 10.1111/jhn.13273. Epub 2024 Jan 7. J Hum Nutr Diet. 2025. PMID: 38185902 Free PMC article. Review.
Cited by
-
A new childhood ALL case with an extremely complex karyotype and acute spontaneous tumor lysis syndrome.Mol Cytogenet. 2020 Sep 11;13:44. doi: 10.1186/s13039-020-00512-3. eCollection 2020. Mol Cytogenet. 2020. PMID: 32944079 Free PMC article.
-
Clinical Epigenomic Explanation of the Epidemiology of Cannabinoid Genotoxicity Manifesting as Transgenerational Teratogenesis, Cancerogenesis and Aging Acceleration.Int J Environ Res Public Health. 2023 Feb 14;20(4):3360. doi: 10.3390/ijerph20043360. Int J Environ Res Public Health. 2023. PMID: 36834053 Free PMC article. Review.
-
Poor Prognosis Biomolecular Factors Are Highly Frequent in Childhood Acute Leukemias From Oaxaca, Mexico.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820928436. doi: 10.1177/1533033820928436. Technol Cancer Res Treat. 2020. PMID: 32608319 Free PMC article.
-
The "Never-Ending" Mouse Models for MLL-Rearranged Acute Leukemia Are Still Teaching Us.Hemasphere. 2018 Jun 19;2(4):e57. doi: 10.1097/HS9.0000000000000057. eCollection 2018 Aug. Hemasphere. 2018. PMID: 31723783 Free PMC article. No abstract available.
-
LncRNA-mRNA Co-Expression Analysis Identifies AL133346.1/CCN2 as Biomarkers in Pediatric B-Cell Acute Lymphoblastic Leukemia.Cancers (Basel). 2020 Dec 17;12(12):3803. doi: 10.3390/cancers12123803. Cancers (Basel). 2020. PMID: 33348573 Free PMC article.
References
-
- Yeoh E-J, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R, Behm FG, Raimondi SC, Relling MV, Patel A, Cheng C, Campana D, Wilkins D, Zhou X, Li J, Liu H, Pui C-H, Evans WE, Naeve C, Wong L, Downing JR. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1(2):133–143. doi: 10.1016/S1535-6108(02)00032-6. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

