Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir
- PMID: 28811349
- PMCID: PMC5559639
- DOI: 10.1128/mBio.01186-17
Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir
Abstract
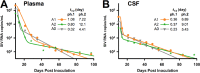
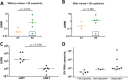
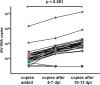
A human immunodeficiency virus (HIV) infection cure requires an understanding of the cellular and anatomical sites harboring virus that contribute to viral rebound upon treatment interruption. Despite antiretroviral therapy (ART), HIV-associated neurocognitive disorders (HAND) are reported in HIV-infected individuals on ART. Biomarkers for macrophage activation and neuronal damage in cerebrospinal fluid (CSF) of HIV-infected individuals demonstrate continued effects of HIV in brain and suggest that the central nervous system (CNS) may serve as a viral reservoir. Using a simian immunodeficiency virus (SIV)/macaque model for HIV encephalitis and AIDS, we evaluated whether infected cells persist in brain despite ART. Eight SIV-infected pig-tailed macaques were virally suppressed with ART, and plasma and CSF viremia levels were analyzed longitudinally. To assess whether virus persisted in brain macrophages (BrMΦ) in these macaques, we used a macrophage quantitative viral outgrowth assay (MΦ-QVOA), PCR, and in situ hybridization (ISH) to measure the frequency of infected cells and the levels of viral RNA and DNA in brain. Viral RNA in brain tissue of suppressed macaques was undetectable, although viral DNA was detected in all animals. The MΦ-QVOA demonstrated that the majority of suppressed animals contained latently infected BrMΦ. We also showed that virus produced in the MΦ-QVOAs was replication competent, suggesting that latently infected BrMΦ are capable of reestablishing productive infection upon treatment interruption. This report provides the first confirmation of the presence of replication-competent SIV in BrMΦ of ART-suppressed macaques and suggests that the highly debated issue of viral latency in macrophages, at least in brain, has been addressed in SIV-infected macaques treated with ART.IMPORTANCE Resting CD4+ T cells are currently the only cells that fit the definition of a latent reservoir. However, recent evidence suggests that HIV/SIV-infected macrophages persist despite ART. Markers of macrophage activation and neuronal damage are observed in the CSF of HIV-infected individuals and of SIV-infected macaques on suppressive ART regimens, suggesting that the CNS has continued virus infection and latent infection. A controversy exists as to whether brain macrophages represent a latent source of replication-competent virus capable of reestablishing infection upon treatment interruption. In this study, we demonstrated the presence of the latent macrophage reservoir in brains of SIV-infected ART-treated macaques and analyzed the reservoir using our established outgrowth assay to quantitate macrophages harboring replication-competent SIV genomes. Our results support the idea of the existence of other latent reservoirs in addition to resting CD4+ T cells and underscore the importance of macrophages in developing strategies to eradicate HIV.
Keywords: brain; human immunodeficiency virus; latency; macrophages; simian immunodeficiency virus.
Copyright © 2017 Avalos et al.
Figures
Similar articles
-
Infectious Virus Persists in CD4+ T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques.J Virol. 2019 Jul 17;93(15):e00065-19. doi: 10.1128/JVI.00065-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31118264 Free PMC article.
-
Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques.mBio. 2019 Aug 20;10(4):e01659-19. doi: 10.1128/mBio.01659-19. mBio. 2019. PMID: 31431552 Free PMC article.
-
Brain macrophages harbor latent, infectious simian immunodeficiency virus.AIDS. 2019 Dec 1;33 Suppl 2(Suppl 2):S181-S188. doi: 10.1097/QAD.0000000000002269. AIDS. 2019. PMID: 31789817 Free PMC article. Review.
-
Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques.J Virol. 2016 May 27;90(12):5643-5656. doi: 10.1128/JVI.00290-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27030272 Free PMC article.
-
An SIV/macaque model targeted to study HIV-associated neurocognitive disorders.J Neurovirol. 2018 Apr;24(2):204-212. doi: 10.1007/s13365-017-0582-4. Epub 2017 Oct 3. J Neurovirol. 2018. PMID: 28975505 Free PMC article. Review.
Cited by
-
The Interplay of HIV-1 and Macrophages in Viral Persistence.Front Microbiol. 2021 Apr 7;12:646447. doi: 10.3389/fmicb.2021.646447. eCollection 2021. Front Microbiol. 2021. PMID: 33897659 Free PMC article. Review.
-
Role of T Lymphocytes in HIV Neuropathogenesis.Curr HIV/AIDS Rep. 2019 Jun;16(3):236-243. doi: 10.1007/s11904-019-00445-6. Curr HIV/AIDS Rep. 2019. PMID: 31062168 Free PMC article. Review.
-
Impact of Myeloid Reservoirs in HIV Cure Trials.Curr HIV/AIDS Rep. 2019 Apr;16(2):129-140. doi: 10.1007/s11904-019-00438-5. Curr HIV/AIDS Rep. 2019. PMID: 30835045 Free PMC article. Review.
-
Curing HIV: Seeking to Target and Clear Persistent Infection.Cell. 2020 Apr 2;181(1):189-206. doi: 10.1016/j.cell.2020.03.005. Epub 2020 Mar 26. Cell. 2020. PMID: 32220311 Free PMC article. Review.
-
Effects of Amprenavir on HIV-1 Maturation, Production and Infectivity Following Drug Withdrawal in Chronically-Infected Monocytes/Macrophages.Viruses. 2017 Sep 28;9(10):277. doi: 10.3390/v9100277. Viruses. 2017. PMID: 28956865 Free PMC article.
References
-
- Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I; CHARTER Group . 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096. doi:10.1212/WNL.0b013e318200d727. - DOI - PMC - PubMed
-
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I; CHARTER Group, HNRC Group . 2011. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16. doi:10.1007/s13365-010-0006-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
