Plasma cell and serum antibody responses to influenza vaccine in preterm and full-term infants
- PMID: 28807607
- PMCID: PMC5603201
- DOI: 10.1016/j.vaccine.2017.07.115
Plasma cell and serum antibody responses to influenza vaccine in preterm and full-term infants
Abstract
Background: Preterm (PT) infants are at greater risk for severe influenza infection and experience decrements in long-term antibody responses to vaccines. This may related to defects in antibody secreting cell (ASC) generation.
Objective: To investigate the relationships among the frequencies of influenza-specific antibody secreting cells, ASC numbers and subsets, and antibody responses to influenza vaccines (IV) among PT and full-term (FT) infants.
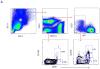
Design/methods: We enrolled 11 former PT (≤32weeks' gestation, ≤1500 g' birth weight) and 11FT infants, 6-17months of age, receiving their first influenza immunizations. Infants received two doses of inactivated trivalent (T)IV or quadrivalent (Q)IV during the 2012-2013 and 2013-2014 influenza seasons, respectively, at 0 and 28days, and blood was drawn at 0, 10, 35, and 56days and 9months. Vaccine-specific antibody was measured by hemagglutination inhibition (HAI) at 0 and 56days and 9months, vaccine-specific ASC numbers by enzyme linked immunospot (ELISPOT) at 10 and 35days, and ASC subsets by flow cytometry at 0, 10 and 35days.
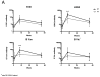
Results: PT infants had post-vaccine HAI titers to all 4 vaccine strains at least equal to FT infants at 56days and 9months after beginning immunization. Influenza-specific ASC ELISPOT responses at 35days were higher among PT than FT infants (median 100 v. 30 per 106 PBMC, p=0.04). ASC numbers at 35days were positively correlated with serum HAI titers at 56days (ρ=0.50-0.80). There were no statistical differences between PT and FT infants in the frequency of five ASC subsets and no specific ASC subset correlated with durability of serum antibody titers.
Conclusions: Influenza-specific ASC numbers in both FT and PT infants correlated with peak antibody titers, but ASC subsets did not correlate with durability of antibody response.
Keywords: Antibody titer; Antibody-secreting cell; Influenza vaccine; Plasma cell; Premature infant.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures







Similar articles
-
Immunogenicity of trivalent influenza vaccine in extremely low-birth-weight, premature versus term infants.Pediatr Infect Dis J. 2011 Jul;30(7):570-4. doi: 10.1097/INF.0b013e31820c1fdf. Pediatr Infect Dis J. 2011. PMID: 21273938 Free PMC article.
-
Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization.Vaccine. 2010 Apr 30;28(20):3582-7. doi: 10.1016/j.vaccine.2010.02.088. Epub 2010 Mar 16. Vaccine. 2010. PMID: 20298818 Free PMC article.
-
Frequencies of human influenza-specific antibody secreting cells or plasmablasts post vaccination from fresh and frozen peripheral blood mononuclear cells.J Immunol Methods. 2009 Jan 1;340(1):42-7. doi: 10.1016/j.jim.2008.09.025. Epub 2008 Nov 6. J Immunol Methods. 2009. PMID: 18996127 Free PMC article.
-
Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers.Pediatrics. 2006 Sep;118(3):e579-85. doi: 10.1542/peds.2006-0201. Pediatrics. 2006. PMID: 16950949 Clinical Trial.
-
Active immunization of premature and low birth-weight infants: a review of immunogenicity, efficacy, and tolerability.Paediatr Drugs. 2007;9(1):17-32. doi: 10.2165/00148581-200709010-00003. Paediatr Drugs. 2007. PMID: 17291134 Review.
Cited by
-
The Importance of Vaccinating Children and Pregnant Women against Influenza Virus Infection.Pathogens. 2019 Nov 26;8(4):265. doi: 10.3390/pathogens8040265. Pathogens. 2019. PMID: 31779153 Free PMC article. Review.
-
An update on vaccination in preterm infants.J Pediatr (Rio J). 2023 Mar-Apr;99 Suppl 1(Suppl 1):S81-S86. doi: 10.1016/j.jped.2022.12.004. Epub 2023 Jan 3. J Pediatr (Rio J). 2023. PMID: 36608935 Free PMC article. Review.
-
CD4+ TSCMs in the Bone Marrow Assist in Maturation of Antibodies against Influenza in Mice.Mediators Inflamm. 2019 Jan 10;2019:3231696. doi: 10.1155/2019/3231696. eCollection 2019. Mediators Inflamm. 2019. PMID: 30733641 Free PMC article.
-
Influenza in Children.Cold Spring Harb Perspect Med. 2021 Jan 4;11(1):a038430. doi: 10.1101/cshperspect.a038430. Cold Spring Harb Perspect Med. 2021. PMID: 31871228 Free PMC article. Review.
-
Differences in Influenza-Specific CD4 T-Cell Mediated Immunity Following Acute Infection Versus Inactivated Vaccination in Children.J Infect Dis. 2021 Jun 15;223(12):2164-2173. doi: 10.1093/infdis/jiaa664. J Infect Dis. 2021. PMID: 33074330 Free PMC article.
References
-
- Resch B, Kurath-Koller S, Eibisberger M, Zenz W. Prematurity and the burden of influenza and respiratory syncytial virus disease. World J Pediatr. 2016;12:8–18. - PubMed
-
- Washburn LK, O’Shea TM, Gillis DC, Block SM, Abramson JS. Response to Haemophilus influenzae type b conjugate vaccine in chronically ill premature infants. Journal of Pediatrics. 1993;123:791–4. - PubMed
-
- Greenberg DP, Vadheim CM, Partridge S, Chang SJ, Chiu CY, Ward JI. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. Journal of Infectious Diseases. 1994;170:76–81. - PubMed
-
- Munoz A, Salvador A, Brodsky NL, Arbeter AM, Porat R. Antibody response of low birth weight infants to Haemophilus influenzae type b polyribosylribitol phosphate-outer membrane protein conjugate vaccine. Pediatrics. 1995;96:216–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

