Modeling of TREX1-Dependent Autoimmune Disease using Human Stem Cells Highlights L1 Accumulation as a Source of Neuroinflammation
- PMID: 28803918
- PMCID: PMC5591075
- DOI: 10.1016/j.stem.2017.07.009
Modeling of TREX1-Dependent Autoimmune Disease using Human Stem Cells Highlights L1 Accumulation as a Source of Neuroinflammation
Abstract
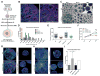
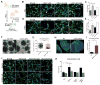
Three-prime repair exonuclease 1 (TREX1) is an anti-viral enzyme that cleaves nucleic acids in the cytosol, preventing accumulation and a subsequent type I interferon-associated inflammatory response. Autoimmune diseases, including Aicardi-Goutières syndrome (AGS) and systemic lupus erythematosus, can arise when TREX1 function is compromised. AGS is a neuroinflammatory disorder with severe and persistent intellectual and physical problems. Here we generated a human AGS model that recapitulates disease-relevant phenotypes using pluripotent stem cells lacking TREX1. We observed abundant extrachromosomal DNA in TREX1-deficient neural cells, of which endogenous Long Interspersed Element-1 retrotransposons were a major source. TREX1-deficient neurons also exhibited increased apoptosis and formed three-dimensional cortical organoids of reduced size. TREX1-deficient astrocytes further contributed to the observed neurotoxicity through increased type I interferon secretion. In this model, reverse-transcriptase inhibitors rescued the neurotoxicity of AGS neurons and organoids, highlighting their potential utility in therapeutic regimens for AGS and related disorders.
Keywords: Aicardi-Goutières syndrome; LINE-1; TREX1; disease modeling; neuroinflammation; type I IFN.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Comment in
-
The TREX1 Dinosaur Bites the Brain through the LINE.Cell Stem Cell. 2017 Sep 7;21(3):287-288. doi: 10.1016/j.stem.2017.08.010. Cell Stem Cell. 2017. PMID: 28886359
Similar articles
-
The TREX1 double-stranded DNA degradation activity is defective in dominant mutations associated with autoimmune disease.J Biol Chem. 2008 Nov 14;283(46):31649-56. doi: 10.1074/jbc.M806155200. Epub 2008 Sep 18. J Biol Chem. 2008. PMID: 18805785 Free PMC article.
-
Lack of Trex1 Causes Systemic Autoimmunity despite the Presence of Antiretroviral Drugs.J Immunol. 2017 Oct 1;199(7):2261-2269. doi: 10.4049/jimmunol.1700714. Epub 2017 Aug 23. J Immunol. 2017. PMID: 28835460
-
Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases.Proc Natl Acad Sci U S A. 2015 Oct 20;112(42):E5699-705. doi: 10.1073/pnas.1516465112. Epub 2015 Sep 14. Proc Natl Acad Sci U S A. 2015. PMID: 26371324 Free PMC article.
-
Aicardi-Goutières syndrome (AGS).Eur J Paediatr Neurol. 2008 Sep;12(5):355-8. doi: 10.1016/j.ejpn.2007.11.010. Epub 2008 Mar 14. Eur J Paediatr Neurol. 2008. PMID: 18343173 Review.
-
T-Rex escaped from the cytosolic park: Re-thinking the impact of TREX1 exonuclease deficiencies on genomic stability.Bioessays. 2024 Aug;46(8):e2400066. doi: 10.1002/bies.202400066. Epub 2024 Jun 4. Bioessays. 2024. PMID: 38837436 Review.
Cited by
-
Brainstem Organoids From Human Pluripotent Stem Cells.Front Neurosci. 2020 Jun 26;14:538. doi: 10.3389/fnins.2020.00538. eCollection 2020. Front Neurosci. 2020. PMID: 32670003 Free PMC article.
-
Brain organoids and insights on human evolution.F1000Res. 2019 May 30;8:F1000 Faculty Rev-760. doi: 10.12688/f1000research.18495.1. eCollection 2019. F1000Res. 2019. PMID: 31275562 Free PMC article. Review.
-
The Sophisticated Transcriptional Response Governed by Transposable Elements in Human Health and Disease.Int J Mol Sci. 2020 Apr 30;21(9):3201. doi: 10.3390/ijms21093201. Int J Mol Sci. 2020. PMID: 32366056 Free PMC article. Review.
-
The transcription factor PAX5 activates human LINE1 retrotransposons to induce cellular senescence.EMBO Rep. 2024 Aug;25(8):3263-3275. doi: 10.1038/s44319-024-00176-9. Epub 2024 Jun 12. EMBO Rep. 2024. PMID: 38866979 Free PMC article.
-
Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention.Signal Transduct Target Ther. 2021 Jun 28;6(1):245. doi: 10.1038/s41392-021-00646-9. Signal Transduct Target Ther. 2021. PMID: 34176928 Free PMC article. Review.
References
-
- Akwa Y, Hassett DE, Eloranta ML, Sandberg K, Masliah E, Powell H, Whitton JL, Bloom FE, Campbell IL. Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161:5016–5026. - PubMed
-
- Arad U. Modified Hirt procedure for rapid purification of extrachromosomal DNA from mammalian cells. Biotechniques. 1998;24:760–762. - PubMed
-
- Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

