A dynamic ribosomal biogenesis response is not required for IGF-1-mediated hypertrophy of human primary myotubes
- PMID: 28774889
- PMCID: PMC5690393
- DOI: 10.1096/fj.201700329R
A dynamic ribosomal biogenesis response is not required for IGF-1-mediated hypertrophy of human primary myotubes
Abstract
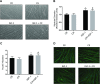
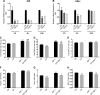
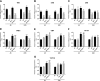
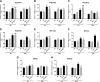
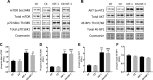
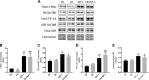
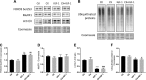
Increased ribosomal DNA transcription has been proposed to limit muscle protein synthesis, making ribosome biogenesis central to skeletal muscle hypertrophy. We examined the relationship between ribosomal RNA (rRNA) production and IGF-1-mediated myotube hypertrophy in vitro Primary skeletal myotubes were treated with IGF-1 (50 ng/ml) with or without 0.5 µM CX-5461 (CX), an inhibitor of RNA polymerase I. Myotube diameter, total protein, and RNA and DNA levels were measured along with markers of RNA polymerase I regulatory factors and regulators of protein synthesis. CX treatment reduced 45S pre-rRNA expression (-64 ± 5% vs. IGF-1; P < 0.001) and total RNA content (-16 ± 2% vs. IGF-1; P < 0.001) in IGF-1-treated myotubes. IGF-1-mediated increases in myotube diameter (1.27 ± 0.09-fold, P < 0.05 vs. control) and total protein (+20 ± 2%; P < 0.001 vs. control) were not prevented by CX treatment. Suppression of rRNA synthesis during IGF-1 treatment did not prevent early increases in AKT (+203 ± 39% vs. CX; P < 0.001) and p70 S6K1 (269 ± 41% vs. CX; P < 0.001) phosphorylation. Despite robust inhibition of the dynamic ribosomal biogenesis response to IGF-1, myotube diameter and protein accretion were sustained. Thus, while ribosome biogenesis represents a potential site for the regulation of skeletal muscle protein synthesis and muscle mass, it does not appear to be a prerequisite for IGF-1-induced myotube hypertrophy in vitro.-Crossland, H., Timmons, J. A., Atherton, P. J. A dynamic ribosomal biogenesis response is not required for IGF-1-mediated hypertrophy of human primary myotubes.
Keywords: RNA; mTOR; skeletal muscle.
© The Author(s).
Figures
Similar articles
-
Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro.Am J Physiol Endocrinol Metab. 2016 Apr 15;310(8):E652-E661. doi: 10.1152/ajpendo.00486.2015. Epub 2016 Feb 9. Am J Physiol Endocrinol Metab. 2016. PMID: 26860985 Free PMC article.
-
Focal adhesion kinase is required for IGF-I-mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1-associated pathway.Am J Physiol Endocrinol Metab. 2013 Jul 15;305(2):E183-93. doi: 10.1152/ajpendo.00541.2012. Epub 2013 May 21. Am J Physiol Endocrinol Metab. 2013. PMID: 23695213 Free PMC article.
-
Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy.Med Sci Sports Exerc. 2013 Sep;45(9):1712-20. doi: 10.1249/MSS.0b013e31828cf5f3. Med Sci Sports Exerc. 2013. PMID: 23470307
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
-
Molecular Mechanisms of Skeletal Muscle Hypertrophy.J Neuromuscul Dis. 2021;8(2):169-183. doi: 10.3233/JND-200568. J Neuromuscul Dis. 2021. PMID: 33216041 Free PMC article. Review.
Cited by
-
Whey Protein Supplementation Effects on Body Composition, Performance, and Blood Biomarkers During Army Initial Entry Training.Front Nutr. 2022 Mar 7;9:807928. doi: 10.3389/fnut.2022.807928. eCollection 2022. Front Nutr. 2022. PMID: 35330708 Free PMC article.
-
Longevity-related molecular pathways are subject to midlife "switch" in humans.Aging Cell. 2019 Aug;18(4):e12970. doi: 10.1111/acel.12970. Epub 2019 Jun 6. Aging Cell. 2019. PMID: 31168962 Free PMC article.
-
Regulation of Ribosome Biogenesis in Skeletal Muscle Hypertrophy.Physiology (Bethesda). 2019 Jan 1;34(1):30-42. doi: 10.1152/physiol.00034.2018. Physiology (Bethesda). 2019. PMID: 30540235 Free PMC article. Review.
-
Ribosome biogenesis restricts innate immune responses to virus infection and DNA.Elife. 2019 Dec 16;8:e49551. doi: 10.7554/eLife.49551. Elife. 2019. PMID: 31841110 Free PMC article.
-
Ribosome accumulation during early phase resistance training in humans.Acta Physiol (Oxf). 2022 May;235(1):e13806. doi: 10.1111/apha.13806. Epub 2022 Mar 7. Acta Physiol (Oxf). 2022. PMID: 35213791 Free PMC article.
References
-
- Fromont-Racine M., Senger B., Saveanu C., Fasiolo F. (2003) Ribosome assembly in eukaryotes. Gene 313, 17–42 - PubMed
-
- Kusnadi E. P., Hannan K. M., Hicks R. J., Hannan R. D., Pearson R. B., Kang J. (2015) Regulation of rDNA transcription in response to growth factors, nutrients and energy. Gene 556, 27–34 - PubMed
-
- Mayer C., Grummt I. (2006) Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25, 6384–6391 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

