Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents
- PMID: 28770976
- PMCID: PMC6460508
- DOI: 10.1002/14651858.CD012537.pub2
Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents
Abstract
Background: Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization guidelines for pharmacological treatments for children's persisting pain acknowledge that pain in children is a major public health concern of high significance in most parts of the world. While in the past pain was largely dismissed and was frequently left untreated, views on children's pain have changed over time, and relief of pain is now seen as important.We designed a suite of seven reviews on chronic non-cancer pain and cancer pain (looking at antidepressants, antiepileptic drugs, non-steroidal anti-inflammatory drugs, opioids, and paracetamol) in order to review the evidence for children's pain utilising pharmacological interventions.As the leading cause of morbidity in the world today, chronic disease (and its associated pain) is a major health concern. Chronic pain (that is pain lasting three months or longer) can arise in the paediatric population in a variety of pathophysiological classifications (nociceptive, neuropathic, or idiopathic) from genetic conditions, nerve damage pain, chronic musculoskeletal pain, and chronic abdominal pain, as well as for other unknown reasons.Non-steroidal anti-inflammatory drugs (NSAIDs) are used to treat pain, reduce fever, and for their anti-inflammation properties. They are commonly used within paediatric pain management. Non-steroidal anti-inflammatory drugs are currently licensed for use in Western countries, however they are not approved for infants under three months old. The main adverse effects include renal impairment and gastrointestinal issues. Common side effects in children include diarrhoea, headache, nausea, constipation, rash, dizziness, and abdominal pain.
Objectives: To assess the analgesic efficacy and adverse events of NSAIDs used to treat chronic non-cancer pain in children and adolescents aged between birth and 17 years, in any setting.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online, MEDLINE via Ovid, and Embase via Ovid from inception to 6 September 2016. We also searched the reference lists of retrieved studies and reviews, as well as online clinical trial registries.
Selection criteria: Randomised controlled trials, with or without blinding, of any dose and any route, treating chronic non-cancer pain in children and adolescents, comparing any NSAID with placebo or an active comparator.
Data collection and analysis: Two review authors independently assessed studies for eligibility. We planned to use dichotomous data to calculate risk ratio and number needed to treat for one additional event, using standard methods. We assessed GRADE and created three 'Summary of findings' tables.
Main results: We included seven studies with a total of 1074 participants (aged 2 to 18 years) with chronic juvenile polyarthritis or chronic juvenile rheumatoid arthritis. All seven studies compared an NSAID with an active comparator. None of the studies were placebo controlled. No two studies investigated the same type of NSAID compared with another. We were unable to perform a meta-analysis.Risk of bias varied. For randomisation and allocation concealment, one study was low risk and six studies were unclear risk. For blinding of participants and personnel, three studies were low risk and four studies were unclear to high risk. For blinding of outcome assessors, all studies were unclear risk. For attrition, four studies were low risk and three studies were unclear risk. For selective reporting, four studies were low risk, two studies were unclear risk, and one study was high risk. For size, three studies were unclear risk and four studies were high risk. For other potential sources of bias, seven studies were low risk. Primary outcomesThree studies reported participant-reported pain relief of 30% or greater, showing no statistically significant difference in pain scores between meloxicam and naproxen, celecoxib and naproxen, or rofecoxib and naproxen (P > 0.05) (low-quality evidence).One study reported participant-reported pain relief of 50% or greater, showing no statistically significant difference in pain scores between low-dose meloxicam (0.125 mg/kg) and high-dose meloxicam (0.25 mg/kg) when compared to naproxen 10 mg/kg (P > 0.05) (low-quality evidence).One study reported Patient Global Impression of Change, showing 'very much improved' in 85% of ibuprofen and 90% of aspirin participants (low-quality evidence). Secondary outcomesAll seven studies reported adverse events. Participants reporting an adverse event (one or more per person) by drug were: aspirin 85/202; fenoprofen 28/49; ibuprofen 40/45; indomethacin 9/30; ketoprofen 9/30; meloxicam 18/47; naproxen 44/202; and rofecoxib 47/209 (very low-quality evidence).All seven studies reported withdrawals due to adverse events. Participants withdrawn due to an adverse event by drug were: aspirin 16/120; celecoxib 10/159; fenoprofen 0/49; ibuprofen 0/45; indomethacin 0/30; ketoprofen 0/30; meloxicam 10/147; naproxen 17/285; and rofecoxib 3/209 (very low-quality evidence).All seven studies reported serious adverse events. Participants experiencing a serious adverse event by drug were: aspirin 13/120; celecoxib 5/159; fenoprofen 0/79; ketoprofen 0/30; ibuprofen 4/45; indomethacin 0/30; meloxicam 11/147; naproxen 10/285; and rofecoxib 0/209 (very low-quality evidence).There were few or no data for our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; sleep duration and quality; acceptability of treatment; physical functioning as defined by validated scales; and quality of life as defined by validated scales (very low-quality evidence).We rated the overall quality of the evidence (GRADE rating) for our primary and secondary outcomes as very low because there were limited data from studies and no opportunity for a meta-analysis.
Authors' conclusions: We identified only a small number of studies, with insufficient data for analysis.As we could undertake no meta-analysis, we are unable to comment about efficacy or harm from the use of NSAIDs to treat chronic non-cancer pain in children and adolescents. Similarly, we cannot comment on our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; sleep duration and quality; acceptability of treatment; physical functioning; and quality of life.We know from adult randomised controlled trials that some NSAIDs, such as ibuprofen, naproxen, and aspirin, can be effective in certain chronic pain conditions.
Conflict of interest statement
CE: none known. Since CE is an author as well as the PaPaS Co‐ordinating Editor at the time of writing, we acknowledge the input of Andrew Moore who acted as Sign Off Editor for this review. CE had no input into the editorial decisions or processes for this review.
TC: none known.
BA: none known; BA is a specialist anaesthetist and intensive care physician and manages the perioperative care of children requiring surgery and those critically ill requiring intensive care.
EF: none known.
NW: none known; NW is a specialist paediatric rheumatologist and treats patients with chronic pain.
Figures
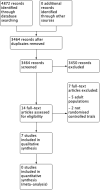


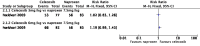
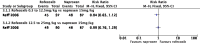





Update of
- doi: 10.1002/14651858.CD012537
Similar articles
-
Antidepressants for chronic non-cancer pain in children and adolescents.Cochrane Database Syst Rev. 2017 Aug 5;8(8):CD012535. doi: 10.1002/14651858.CD012535.pub2. Cochrane Database Syst Rev. 2017. PMID: 28779487 Free PMC article. Review.
-
Antiepileptic drugs for chronic non-cancer pain in children and adolescents.Cochrane Database Syst Rev. 2017 Aug 5;8(8):CD012536. doi: 10.1002/14651858.CD012536.pub2. Cochrane Database Syst Rev. 2017. PMID: 28779491 Free PMC article. Review.
-
Non-steroidal anti-inflammatory drugs (NSAIDs) for cancer-related pain in children and adolescents.Cochrane Database Syst Rev. 2017 Jul 24;7(7):CD012563. doi: 10.1002/14651858.CD012563.pub2. Cochrane Database Syst Rev. 2017. PMID: 28737843 Free PMC article. Review.
-
Opioids for chronic non-cancer pain in children and adolescents.Cochrane Database Syst Rev. 2017 Jul 26;7(7):CD012538. doi: 10.1002/14651858.CD012538.pub2. Cochrane Database Syst Rev. 2017. PMID: 28745394 Free PMC article. Review.
-
Paracetamol (acetaminophen) for chronic non-cancer pain in children and adolescents.Cochrane Database Syst Rev. 2017 Aug 2;8(8):CD012539. doi: 10.1002/14651858.CD012539.pub2. Cochrane Database Syst Rev. 2017. PMID: 28770975 Free PMC article. Review.
Cited by
-
The Emerging Role of Quercetin in the Treatment of Chronic Pain.Curr Neuropharmacol. 2022 Nov 15;20(12):2346-2353. doi: 10.2174/1570159X20666220812122437. Curr Neuropharmacol. 2022. PMID: 35959909 Free PMC article. Review.
-
Treatment of Unspecific Back Pain in Children and Adolescents: Results of an Evidence-Based Interdisciplinary Guideline.Children (Basel). 2022 Mar 15;9(3):417. doi: 10.3390/children9030417. Children (Basel). 2022. PMID: 35327789 Free PMC article. Review.
-
Over-the-counter analgesics use is associated with pain and psychological distress among adolescents: a mixed effects approach in cross-sectional survey data from Norway.BMC Public Health. 2021 Nov 6;21(1):2030. doi: 10.1186/s12889-021-12054-3. BMC Public Health. 2021. PMID: 34742281 Free PMC article.
-
Antidepressants for chronic non-cancer pain in children and adolescents.Cochrane Database Syst Rev. 2017 Aug 5;8(8):CD012535. doi: 10.1002/14651858.CD012535.pub2. Cochrane Database Syst Rev. 2017. PMID: 28779487 Free PMC article. Review.
-
Therapeutic Application of Exosomes in Inflammatory Diseases.Int J Mol Sci. 2021 Jan 24;22(3):1144. doi: 10.3390/ijms22031144. Int J Mol Sci. 2021. PMID: 33498928 Free PMC article. Review.
References
References to studies included in this review
Bhettay 1978 {published data only}
-
- Bhettay E, Thomson AJG. Double‐blind study of ketoprofen and indomethacin in juvenile chronic arthritis. South African Medical Journal 1978;54(7):276‐8. - PubMed
Brewer 1982 {published data only}
-
- Brewer EJ, Giannini EH, Baum J. Aspirin and fenoprofen (Nalfon) in the treatment of juvenile rheumatoid arthritis results of the double blind‐trial. A segment II study. Journal of Rheumatology 1982;9(1):123‐8. - PubMed
Foeldvari 2009 {published data only}
-
- Foeldvari I, Szer IS, Zemel LS, Lovell DJ, Giannini EH, Robbins JL, et al. A prospective study comparing celecoxib with naproxen in children with juvenile rheumatoid arthritis. Journal of Rheumatology 2009;36(1):174‐82. - PubMed
Giannini 1990 {published data only}
-
- Giannini EH, Brewer EJ, Miller M L, Gibbas D, Passo MH, Hoyeraal HM. Ibuprofen suspension in the treatment of juvenile rheumatoid arthritis. Journal of Pediatrics 1990;117(4):645‐52. - PubMed
Moran 1979 {published data only}
Reiff 2006 {published data only}
-
- Reiff A, Lovell DJ, Adelsberg J, Kiss MHB, Goodman S, Zavaler MF, et al. Evaluation of the comparative efficacy and tolerability of rofecoxib and naproxen in children and adolescents with juvenile rheumatoid arthritis: a 12‐week randomized controlled clinical trial with a 52‐week open‐label extension. Journal of Rheumatology 2006;33(5):985‐95. - PubMed
Ruperto 2005 {published data only}
-
- Ruperto N, Nikishina I, Pachanov ED, Shachbazian Y, Prieur AM, Mouy R, et al. A randomized, double‐blind clinical trial of two doses of meloxicam compared with naproxen in children with juvenile idiopathic arthritis. Arthritis & Rheumatism 2005;52(2):563‐72. - PubMed
References to studies excluded from this review
Coutinho 1976 {published data only}
-
- Coutinho A, Bonello J, Carvalho PC. A double‐blind comparative study of the analgesic effects of fenbufen, codeine, aspirin, propoxyphene and placebo. Current Therapeutic Research, Clinical and Experimental 1976;19(1):58‐65. - PubMed
Girschick 1999 {published data only}
-
- Girschick HJ, Seyberth HW, Huppertz HI. Treatment of childhood hypophosphatasia with nonsteroidal antiinflammatory drugs. Bone 1999;25(5):603‐7. - PubMed
Jenkins 1976 {published data only}
-
- Jenkins DG, Ebbutt AF, Evans CD. Tofranil in the treatment of low back pain. Journal of International Medical Research 1976;4(2 Suppl):28‐40. - PubMed
Johnsen 1992 {published data only}
-
- Johnsen V, Brun JG, Fjeld E, Hansen K, Sydnes OA, Ugstad MB. Morning stiffness and nighttime pain in ankylosing spondylitis. A comparison between enteric‐coated and plain naproxen tablets. European Journal of Rheumatology and Inflammation 1992;12(2):37‐42. - PubMed
Natour 2002 {published data only}
-
- Natour J, Puertas EB, Radu AS, Freire M, Bonfigliolo R, Schincaroil NRB, et al. Loxoprofen in the treatment of low back pain ‐ clinical efficacy and safety in comparison to diclofenac. Revista Brasileira de Medicina 2002;59(3):161‐70.
Reicher 1969 {published data only}
-
- Reicher E, Reipert D, Kowalczewska J. Results of the treatment of juvenile rheumatoid arthritis with indomethacine. Polskie Archiwum Medycyny Wewnętrznej 1969;42(1):105‐12. - PubMed
Sadowska‐Wroblewska 1980 {published data only}
-
- Sadowska‐Wroblewska M, Garwolinska H, Filipovicz‐Sosnowska A. Azapropazone versus indomethacin in a double blind test with patients with ankylosing spondylitis [Azapropazon versus indometacin im doppelblindversuch bei patienten mit spondylitis ankylosans]. Zeitschrift fur Rheumatologie 1980;39:11‐2. - PubMed
Additional references
AMA 2013
-
- American Medical Association. Pediatric pain management. https://www.ama‐assn.org/ (accessed 25 January 2016).
AUREF 2012
-
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 16 July 2016).
Blanca‐Lopez 2015
-
- Blanca‐López N, Cornejo‐García JA, Plaza‐Serón MC, Doña I, Torres‐Jaén MJ, Canto G, et al. Hypersensitivity to nonsteroidal anti‐inflammatory drugs in children and adolescents: cross‐intolerance reactions. Journal of Investigational Allergology & Clinical Immunology 2015;25(4):259‐69. - PubMed
BNF 2016
-
- Joint Formulary Committee. British National Formulary. London (UK): BMJ Group and Pharmaceutical Press, 2016.
Caes 2016
Calvo 2012
Cooper 2017a
Cooper 2017b
Cooper 2017c
Cooper 2017d
Dechartres 2013
Dechartres 2014
Dworkin 2008
Eccleston 2003
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI: 10.1136/bmj.39489.470347.AD] - DOI - PMC - PubMed
Guyatt 2011
Guyatt 2013a
Guyatt 2013b
Hasnie 2007
-
- Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, et al. Further characterization of a rat model of varicella zoster virus‐associated pain: relationship between mechanical hypersensitivity and anxiety‐related behavior, and the influence of analgesic drugs. Neuroscience 2007;144(4):1495‐508. [DOI: 10.1016/j.neuroscience.2006.11.029] - DOI - PMC - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Hoffman 2010
Kawakami 2002
-
- Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Tamaki T. Epidural injection of cyclooxygenase‐2 inhibitor attenuates pain‐related behavior following application of nucleus pulposus to the nerve root in the rat. Journal of Orthopaedic Research 2002;20(2):376‐81. [DOI: 10.1016/S0736-0266(01)00114-0] - DOI - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
Lesko 1995
-
- Lesko SM, Mitchell AA. An assessment of the safety of pediatric ibuprofen. JAMA 1995;273(12):929‐33. - PubMed
Lesko 1997
-
- Lesko SM, Mitchell AA. Renal function after short‐term ibuprofen use in infants and children. Pediatrics 1997;100(6):954‐7. - PubMed
Lesko 1999
-
- Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics 1999;104(4):e39. - PubMed
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford (UK): Oxford University Press, 1998.
Misurac 2013
Moher 2009
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle (WA): IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2009
Moore 2010a
Moore 2010b
-
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. - PubMed
Moore 2010c
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010d
Moore 2010e
-
- Moore RA, Derry S, McQuay HJ, Straube S, Aldington D, Wiffen P, et al. ACTINPAIN writing group of the IASP Special Interest Group (SIG) on Systematic Reviews in Pain Relief. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain 2010;149:173‐6. [PUBMED: 19748185] - PubMed
Moore 2011a
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] - DOI - PubMed
Moore 2011b
Moore 2012
Moore 2013a
Moore 2013b
Moore 2014a
-
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. - PubMed
Moore 2014b
NICE 2015
-
- National Institute of Health and Care Excellence (NICE). Guidance ‐ sickle cell acute painful episode. https://www.nice.org.uk/guidance/cg143/evidence/full‐guideline‐pdf‐18663... (accessed 7 September 2015).
Nüesch 2010
O'Brien 2010
PedIMMPACT 2008
-
- McGrath PJ, Walco GA, Turk DC, Dworking RH, Brown MT, Davidson K, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT. Journal of Pain 2008;9(9):771‐83. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripamonti 2008
Stinson 2006
-
- Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self‐report pain intensity measures for use in clinical trials in children and adolescents. Pain 2006;125(1‐2):143‐57. [DOI: 10.1016/j.pain.2006.05.006] - DOI - PubMed
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010
Sultan 2008
Thorlund 2011
United Nations 2015
-
- United Nations. World population prospects 2015 ‐ population indicators. esa.un.org/unpd/wpp/Download/Standard/Population/ (accessed 29 February 2016).
Vo 2009
von Baeyer 2007
WHO 2012
-
- World Health Organization. WHO Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses. Geneva: WHO Press, World Health Organization, 2012. [ISBN 978 92 4 154812 0] - PubMed
Wiffen 2017a
Wiffen 2017b
World Bank 2014
-
- World Bank. Data ‐ population ages 0‐14 (% of total). data.worldbank.org/indicator/SP.POP.0014.TO.ZS (accessed 29 February 2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

