Ubiquitin Ligase WWP1 Interacts with Ebola Virus VP40 To Regulate Egress
- PMID: 28768865
- PMCID: PMC5625505
- DOI: 10.1128/JVI.00812-17
Ubiquitin Ligase WWP1 Interacts with Ebola Virus VP40 To Regulate Egress
Abstract
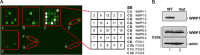
Ebola virus (EBOV) is a member of the Filoviridae family and the cause of hemorrhagic fever outbreaks. The EBOV VP40 (eVP40) matrix protein is the main driving force for virion assembly and budding. Indeed, expression of eVP40 alone in mammalian cells results in the formation and budding of virus-like particles (VLPs) which mimic the budding process and morphology of authentic, infectious EBOV. To complete the budding process, eVP40 utilizes its PPXY L-domain motif to recruit a specific subset of host proteins containing one or more modular WW domains that then function to facilitate efficient production and release of eVP40 VLPs. In this report, we identified additional host WW-domain interactors by screening for potential interactions between mammalian proteins possessing one or more WW domains and WT or PPXY mutant peptides of eVP40. We identified the HECT family E3 ubiquitin ligase WWP1 and all four of its WW domains as strong interactors with the PPXY motif of eVP40. The eVP40-WWP1 interaction was confirmed by both peptide pulldown and coimmunoprecipitation assays, which also demonstrated that modular WW domain 1 of WWP1 was most critical for binding to eVP40. Importantly, the eVP40-WWP1 interaction was found to be biologically relevant for VLP budding since (i) small interfering RNA (siRNA) knockdown of endogenous WWP1 resulted in inhibition of eVP40 VLP egress, (ii) coexpression of WWP1 and eVP40 resulted in ubiquitination of eVP40 and a subsequent increase in eVP40 VLP egress, and (iii) an enzymatically inactive mutant of WWP1 (C890A) did not ubiquitinate eVP40 or enhance eVP40 VLP egress. Last, our data show that ubiquitination of eVP40 by WWP1 enhances egress of VLPs and concomitantly decreases cellular levels of higher-molecular-weight oligomers of eVP40. In sum, these findings contribute to our fundamental understanding of the functional interplay between host E3 ligases, ubiquitination, and regulation of EBOV VP40-mediated egress.IMPORTANCE Ebola virus (EBOV) is a high-priority, emerging human pathogen that can cause severe outbreaks of hemorrhagic fever with high mortality rates. As there are currently no approved vaccines or treatments for EBOV, a better understanding of the biology and functions of EBOV-host interactions that promote or inhibit viral budding is warranted. Here, we describe a physical and functional interaction between EBOV VP40 (eVP40) and WWP1, a host E3 ubiquitin ligase that ubiquitinates VP40 and regulates VLP egress. This viral PPXY-host WW domain-mediated interaction represents a potential new target for host-oriented inhibitors of EBOV egress.
Keywords: E3 ubiquitin ligase; Ebola virus; L-domain; PPXY; VLPs; VP40; WW domain; WWP1; budding.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
ITCH E3 Ubiquitin Ligase Interacts with Ebola Virus VP40 To Regulate Budding.J Virol. 2016 Sep 29;90(20):9163-71. doi: 10.1128/JVI.01078-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489272 Free PMC article.
-
Ubiquitin Ligase SMURF2 Interacts with Filovirus VP40 and Promotes Egress of VP40 VLPs.Viruses. 2021 Feb 12;13(2):288. doi: 10.3390/v13020288. Viruses. 2021. PMID: 33673144 Free PMC article.
-
Angiomotin regulates budding and spread of Ebola virus.J Biol Chem. 2020 Jun 19;295(25):8596-8601. doi: 10.1074/jbc.AC120.013171. Epub 2020 May 7. J Biol Chem. 2020. PMID: 32381509 Free PMC article.
-
Host and Viral Proteins Modulating Ebola and Marburg Virus Egress.Viruses. 2019 Jan 3;11(1):25. doi: 10.3390/v11010025. Viruses. 2019. PMID: 30609802 Free PMC article. Review.
-
Viruses go modular.J Biol Chem. 2020 Apr 3;295(14):4604-4616. doi: 10.1074/jbc.REV119.012414. Epub 2020 Feb 28. J Biol Chem. 2020. PMID: 32111739 Free PMC article. Review.
Cited by
-
Marburg virus exploits the Rab11-mediated endocytic pathway in viral-particle production.Microbiol Spectr. 2024 Sep 3;12(9):e0026924. doi: 10.1128/spectrum.00269-24. Epub 2024 Jul 30. Microbiol Spectr. 2024. PMID: 39078193 Free PMC article.
-
Mutation of Phenylalanine 23 of Newcastle Disease Virus Matrix Protein Inhibits Virus Release by Disrupting the Interaction between the FPIV L-Domain and Charged Multivesicular Body Protein 4B.Microbiol Spectr. 2023 Feb 14;11(1):e0411622. doi: 10.1128/spectrum.04116-22. Epub 2023 Jan 25. Microbiol Spectr. 2023. PMID: 36695580 Free PMC article.
-
A Conserved Tryptophan in the Ebola Virus Matrix Protein C-Terminal Domain Is Required for Efficient Virus-Like Particle Formation.Pathogens. 2020 May 22;9(5):402. doi: 10.3390/pathogens9050402. Pathogens. 2020. PMID: 32455873 Free PMC article.
-
Importance of Viral Late Domains in Budding and Release of Enveloped RNA Viruses.Viruses. 2021 Aug 6;13(8):1559. doi: 10.3390/v13081559. Viruses. 2021. PMID: 34452424 Free PMC article. Review.
-
Molecular insights into the Ebola virus life cycle.Nat Microbiol. 2024 Jun;9(6):1417-1426. doi: 10.1038/s41564-024-01703-z. Epub 2024 May 23. Nat Microbiol. 2024. PMID: 38783022 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

