Age-dependent plasticity in endocannabinoid modulation of pain processing through postnatal development
- PMID: 28767505
- PMCID: PMC5642337
- DOI: 10.1097/j.pain.0000000000001027
Age-dependent plasticity in endocannabinoid modulation of pain processing through postnatal development
Abstract
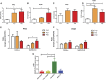
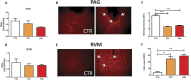
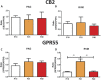
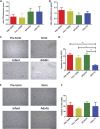
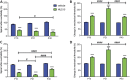
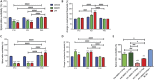
Significant age- and experience-dependent remodelling of spinal and supraspinal neural networks occur, resulting in altered pain responses in early life. In adults, endogenous opioid peptide and endocannabinoid (ECs) pain control systems exist which modify pain responses, but the role they play in acute responses to pain and postnatal neurodevelopment is unknown. Here, we have studied the changing role of the ECs in the brainstem nuclei essential for the control of nociception from birth to adulthood in both rats and humans. Using in vivo electrophysiology, we show that substantial functional changes occur in the effect of microinjection of ECs receptor agonists and antagonists in the periaqueductal grey (PAG) and rostroventral medulla (RVM), both of which play central roles in the supraspinal control of pain and the maintenance of chronic pain states in adulthood. We show that in immature PAG and RVM, the orphan receptor, GPR55, is able to mediate profound analgesia which is absent in adults. We show that tissue levels of endocannabinoid neurotransmitters, anandamide and 2-arachidonoylglycerol, within the PAG and RVM are developmentally regulated (using mass spectrometry). The expression patterns and levels of ECs enzymes and receptors were assessed using quantitative PCR and immunohistochemistry. In human brainstem, we show age-related alterations in the expression of key enzymes and receptors involved in ECs function using PCR and in situ hybridisation. These data reveal that significant changes on ECs that to this point have been unknown and which shed new light into the complex neurochemical changes that permit normal, mature responses to pain.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
Similar articles
-
Postnatal maturation of endogenous opioid systems within the periaqueductal grey and spinal dorsal horn of the rat.Pain. 2014 Jan;155(1):168-178. doi: 10.1016/j.pain.2013.09.022. Epub 2013 Sep 27. Pain. 2014. PMID: 24076162 Free PMC article.
-
Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla.Neuropharmacology. 2005 Dec;49(8):1201-9. doi: 10.1016/j.neuropharm.2005.07.007. Epub 2005 Aug 29. Neuropharmacology. 2005. PMID: 16129456
-
Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors.J Pharmacol Exp Ther. 2006 Mar;316(3):969-82. doi: 10.1124/jpet.105.093286. Epub 2005 Nov 11. J Pharmacol Exp Ther. 2006. PMID: 16284279
-
Endocannabinoid mechanisms of pain modulation.AAPS J. 2006 Nov 17;8(4):E693-708. doi: 10.1208/aapsj080479. AAPS J. 2006. PMID: 17233533 Free PMC article. Review.
-
Endocannabinoid system and pain: an introduction.Proc Nutr Soc. 2014 Feb;73(1):106-17. doi: 10.1017/S0029665113003650. Epub 2013 Oct 22. Proc Nutr Soc. 2014. PMID: 24148358 Review.
Cited by
-
Neonatal complete Freund's adjuvant-induced inflammation does not induce or alter hyperalgesic priming or alter adult distributions of C-fibre dorsal horn innervation.Pain Rep. 2020 Nov 27;5(6):e872. doi: 10.1097/PR9.0000000000000872. eCollection 2020 Nov-Dec. Pain Rep. 2020. PMID: 33274305 Free PMC article.
-
Amygdalar endocannabinoids are affected by predator odor stress in a sex-specific manner and modulate acoustic startle reactivity in female rats.Neurobiol Stress. 2021 Sep 3;15:100387. doi: 10.1016/j.ynstr.2021.100387. eCollection 2021 Nov. Neurobiol Stress. 2021. PMID: 34522703 Free PMC article.
-
Cannabinoids in the descending pain modulatory circuit: Role in inflammation.Pharmacol Ther. 2020 May;209:107495. doi: 10.1016/j.pharmthera.2020.107495. Epub 2020 Jan 29. Pharmacol Ther. 2020. PMID: 32004514 Free PMC article. Review.
-
Cannabis oil extracts for chronic pain: what else can be learned from another structured prospective cohort?Pain Rep. 2024 Apr 26;9(2):e1143. doi: 10.1097/PR9.0000000000001143. eCollection 2024 Apr. Pain Rep. 2024. PMID: 38680212 Free PMC article.
References
-
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol 1978;4:451–62. - PubMed
-
- Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol 1979;187:513–31. - PubMed
-
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 1984;7:309–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

