Inhibition of Aurora Kinase A Induces Necroptosis in Pancreatic Carcinoma
- PMID: 28764929
- PMCID: PMC5670014
- DOI: 10.1053/j.gastro.2017.07.036
Inhibition of Aurora Kinase A Induces Necroptosis in Pancreatic Carcinoma
Abstract
Background & aims: Induction of nonapoptotic cell death could be an approach to eliminate apoptosis-resistant tumors. We investigated necroptosis-based therapies in mouse models of pancreatic ductal adenocarcinoma cancer (PDAC).
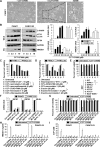
Methods: We screened 273 commercially available kinase inhibitors for cytotoxicity against a human PDAC cell line (PANC1). We evaluated the ability of the aurora kinase inhibitor CCT137690 to stimulate necroptosis in PDAC cell lines (PANC1, PANC2.03, CFPAC1, MiaPaCa2, BxPc3, and PANC02) and the HEK293 cell line, measuring loss of plasma membrane integrity, gain in cell volume, swollen organelles, and cytoplasmic vacuoles. We tested the effects of CCT137690 in colon formation assays, and the effects of the necroptosis (necrostatin-1 and necrosulfonamide), apoptosis, autophagy, and ferroptosis inhibitors. We derived cells from tumors that developed in Pdx1-Cre;K-RasG12D/+;p53R172H/+ (KPC) mice. Genes encoding proteins in cell death pathways were knocked out, knocked down, or expressed from transgenes in PDAC cell lines. Athymic nude or B6 mice were given subcutaneous injections of PDAC cells or tail-vein injections of KPC tumor cells. Mice were given CCT137690 (80 mg/kg) or vehicle and tumor growth was monitored; tumor tissues were collected and analyzed by immunohistochemistry. We compared gene expression levels between human pancreatic cancer tissues (n = 130) with patient survival times using the online R2 genomics analysis and visualization platform.
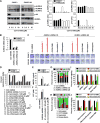
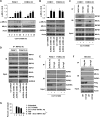
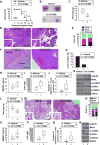
Results: CCT137690 induced necrosis-like death in PDAC cell lines and reduced colony formation; these effects required RIPK1, RIPK3, and MLKL, as well as inhibition of aurora kinase A (AURKA). AURKA interacted directly with RIPK1 and RIPK3 to reduce necrosome activation. AURKA-mediated phosphorylation of glycogen synthase kinase 3 beta (GSK3β) at serine 9 inhibited activation of the RIPK3 and MLKL necrosome. Mutations in AURKA (D274A) or GSK3β (S9A), or pharmacologic inhibitors of RIPK1 signaling via RIPK3 and MLKL, reduced the cytotoxic activity of CCT137690 in PDAC cells. Oral administration of CCT137690 induced necroptosis and immunogenic cell death in subcutaneous and orthotopic tumors in mice, and reduced tumor growth and tumor cell phosphorylation of AURKA and GSK3β. CCT137690 increased survival times of mice with orthotopic KPC PDACs and reduced tumor growth, stroma, and metastasis. Increased expression of AURKA and GSK3β mRNAs associated with shorter survival times of patients with pancreatic cancer.
Conclusions: We identified the aurora kinase inhibitor CCT137690 as an agent that induces necrosis-like death in PDAC cells, via RIPK1, RIPK3, and MLKL. CCT137690 slowed growth of orthotopic tumors from PDAC cells in mice, and expression of AURKA and GSK3β associate with patient survival times. AURKA might be targeted for treatment of pancreatic cancer.
Keywords: ATP; Antitumor Immunity; HMGB1; Regulated Cell Death.
Copyright © 2017 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
JTC801 Induces pH-dependent Death Specifically in Cancer Cells and Slows Growth of Tumors in Mice.Gastroenterology. 2018 Apr;154(5):1480-1493. doi: 10.1053/j.gastro.2017.12.004. Epub 2017 Dec 14. Gastroenterology. 2018. PMID: 29248440 Free PMC article.
-
Regulation of pH by Carbonic Anhydrase 9 Mediates Survival of Pancreatic Cancer Cells With Activated KRAS in Response to Hypoxia.Gastroenterology. 2019 Sep;157(3):823-837. doi: 10.1053/j.gastro.2019.05.004. Epub 2019 May 9. Gastroenterology. 2019. PMID: 31078621
-
The Evaluation of Effect of Aurora Kinase Inhibitor CCT137690 in Melanoma and Melanoma Cancer Stem Cell.Anticancer Agents Med Chem. 2021;21(12):1564-1574. doi: 10.2174/1871520620666201026155101. Anticancer Agents Med Chem. 2021. PMID: 33106151
-
Aurora kinase A, a synthetic lethal target for precision cancer medicine.Exp Mol Med. 2021 May;53(5):835-847. doi: 10.1038/s12276-021-00635-6. Epub 2021 May 28. Exp Mol Med. 2021. PMID: 34050264 Free PMC article. Review.
-
Everolimus for the treatment of advanced pancreatic ductal adenocarcinoma (PDAC).Expert Opin Investig Drugs. 2019 Jul;28(7):583-592. doi: 10.1080/13543784.2019.1632289. Epub 2019 Jun 21. Expert Opin Investig Drugs. 2019. PMID: 31215251 Free PMC article. Review.
Cited by
-
Construction of a Competitive Endogenous RNA Network for Pancreatic Adenocarcinoma Based on Weighted Gene Co-expression Network Analysis and a Prognosis Model.Front Bioeng Biotechnol. 2020 May 28;8:515. doi: 10.3389/fbioe.2020.00515. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32548103 Free PMC article.
-
Lipid metabolism in pancreatic cancer: emerging roles and potential targets.Cancer Commun (Lond). 2022 Dec;42(12):1234-1256. doi: 10.1002/cac2.12360. Epub 2022 Sep 15. Cancer Commun (Lond). 2022. PMID: 36107801 Free PMC article. Review.
-
PINK1 and PARK2 Suppress Pancreatic Tumorigenesis through Control of Mitochondrial Iron-Mediated Immunometabolism.Dev Cell. 2018 Aug 20;46(4):441-455.e8. doi: 10.1016/j.devcel.2018.07.012. Epub 2018 Aug 9. Dev Cell. 2018. PMID: 30100261 Free PMC article.
-
The lipid basis of cell death and autophagy.Autophagy. 2024 Mar;20(3):469-488. doi: 10.1080/15548627.2023.2259732. Epub 2023 Sep 28. Autophagy. 2024. PMID: 37768124 Free PMC article. Review.
-
Targeting AURKA by microRNA-490-3p suppresses gastric cancer cell growth.Histol Histopathol. 2022 Apr;37(4):397-404. doi: 10.14670/HH-18-415. Epub 2021 Dec 24. Histol Histopathol. 2022. PMID: 34951005
References
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. - PubMed
-
- Weinlich R, Oberst A, Beere HM, et al. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2016 - PubMed
-
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

