Flow-Responsive Vascular Endothelial Growth Factor Receptor-Protein Kinase C Isoform Epsilon Signaling Mediates Glycolytic Metabolites for Vascular Repair
- PMID: 28762754
- PMCID: PMC5695747
- DOI: 10.1089/ars.2017.7044
Flow-Responsive Vascular Endothelial Growth Factor Receptor-Protein Kinase C Isoform Epsilon Signaling Mediates Glycolytic Metabolites for Vascular Repair
Abstract
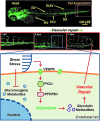
Aims: Hemodynamic shear stress participates in maintaining vascular redox status. Elucidating flow-mediated endothelial metabolites enables us to discover metabolic biomarkers and therapeutic targets. We posited that flow-responsive vascular endothelial growth factor receptor (VEGFR)-protein kinase C isoform epsilon (PKCɛ)-6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) signaling modulates glycolytic metabolites for vascular repair.
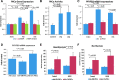
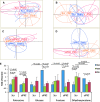
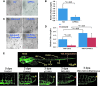
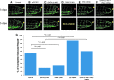
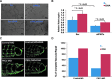
Results: Bidirectional oscillatory flow (oscillatory shear stress [OSS]: 0.1 ± 3 dyne·cm-2 at 1 Hz) upregulated VEGFR-dependent PKCɛ expression to a greater degree than did unidirectional pulsatile flow (pulsatile shear stress [PSS]: 23 ± 8 dyne·cm-2 at 1 Hz) in human aortic endothelial cells (p < 0.05, n = 3). PSS and OSS further upregulated PKCɛ-dependent PFKFB3 expression for glycolysis (p < 0.05, n = 4). Constitutively active PKCɛ increased, whereas dominant-negative PKCɛ reduced both basal and maximal extracellular acidification rates for glycolytic flux (p < 0.01, n = 4). Metabolomic analysis demonstrated an increase in PKCɛ-dependent glycolytic metabolite, dihydroxyacetone (DHA), but a decrease in gluconeogenic metabolite, aspartic acid (p < 0.05 vs. control, n = 6). In a New Zealand White rabbit model, both PKCɛ and PFKFB3 immunostaining was prominent in the PSS- and OSS-exposed aortic arch and descending aorta. In a transgenic Tg(flk-1:EGFP) zebrafish model, GATA-1a morpholino oligonucleotide injection (to reduce viscosity-dependent shear stress) impaired vascular regeneration after tail amputation (p < 0.01, n = 20), which was restored with PKCɛ messenger RNA (mRNA) rescue (p < 0.05, n = 5). As a corollary, siPKCɛ inhibited tube formation and vascular repair, which were restored by DHA treatment in our Matrigel and zebrafish models. Innovation and Conclusion: Flow-sensitive VEGFR-PKCɛ-PFKFB3 signaling increases the glycolytic metabolite, dihydroxyacetone, to promote vascular repair. Antioxid. Redox Signal. 28, 31-43.
Keywords: PFKFB3; PKCɛ; dihydroxyacetone; metabolites; shear stress; vascular repair.
Conflict of interest statement
No competing financial interests exist.
Figures
Similar articles
-
Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):137-45. doi: 10.1161/ATVBAHA.114.304277. Epub 2014 Oct 30. Arterioscler Thromb Vasc Biol. 2015. PMID: 25359860
-
Regulatory role of TIGAR on endothelial metabolism and angiogenesis.J Cell Physiol. 2021 Nov;236(11):7578-7590. doi: 10.1002/jcp.30401. Epub 2021 Apr 30. J Cell Physiol. 2021. PMID: 33928637 Free PMC article.
-
Shear stress-activated Wnt-angiopoietin-2 signaling recapitulates vascular repair in zebrafish embryos.Arterioscler Thromb Vasc Biol. 2014 Oct;34(10):2268-75. doi: 10.1161/ATVBAHA.114.303345. Epub 2014 Aug 21. Arterioscler Thromb Vasc Biol. 2014. PMID: 25147335 Free PMC article.
-
Treatment against glucose-dependent cancers through metabolic PFKFB3 targeting of glycolytic flux.Cancer Metastasis Rev. 2022 Jun;41(2):447-458. doi: 10.1007/s10555-022-10027-5. Epub 2022 Apr 14. Cancer Metastasis Rev. 2022. PMID: 35419769 Review.
-
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 and 4: A pair of valves for fine-tuning of glucose metabolism in human cancer.Mol Metab. 2019 Feb;20:1-13. doi: 10.1016/j.molmet.2018.11.013. Epub 2018 Dec 5. Mol Metab. 2019. PMID: 30553771 Free PMC article. Review.
Cited by
-
The glycolytic process in endothelial cells and its implications.Acta Pharmacol Sin. 2022 Feb;43(2):251-259. doi: 10.1038/s41401-021-00647-y. Epub 2021 Apr 13. Acta Pharmacol Sin. 2022. PMID: 33850277 Free PMC article. Review.
-
Light-sheet Fluorescence Microscopy to Capture 4-Dimensional Images of the Effects of Modulating Shear Stress on the Developing Zebrafish Heart.J Vis Exp. 2018 Aug 10;(138):57763. doi: 10.3791/57763. J Vis Exp. 2018. PMID: 30148501 Free PMC article.
-
Endothelial mechanotransduction in cardiovascular development and regeneration: emerging approaches and animal models.Curr Top Membr. 2021;87:131-151. doi: 10.1016/bs.ctm.2021.07.002. Epub 2021 Oct 12. Curr Top Membr. 2021. PMID: 34696883 Free PMC article. Review.
-
Flow-induced reprogramming of endothelial cells in atherosclerosis.Nat Rev Cardiol. 2023 Nov;20(11):738-753. doi: 10.1038/s41569-023-00883-1. Epub 2023 May 24. Nat Rev Cardiol. 2023. PMID: 37225873 Free PMC article. Review.
-
Advanced microscopy to elucidate cardiovascular injury and regeneration: 4D light-sheet imaging.Prog Biophys Mol Biol. 2018 Oct;138:105-115. doi: 10.1016/j.pbiomolbio.2018.05.003. Epub 2018 May 9. Prog Biophys Mol Biol. 2018. PMID: 29752956 Free PMC article. Review.
References
-
- Parkinson disease. Metablomics study reveals novel biomarkers for PD. Nat Rev Neurol 9: 484, 2013 - PubMed
-
- Stroke. Molecular markers could help predict stroke risk after TIA. Nat Rev Neurol 11: 3, 2015 - PubMed
-
- Bakker JL, Meijers-Heijboer H, and Verheul H. Novel strategies towards the use of anti-angiogenic agents in breast cancer. Eur J Pharmacol 717: 36–39, 2013 - PubMed
-
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, and Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: Role of protein kinase A. J Biol Chem 277: 3388–3396, 2002 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

