Entry, Intracellular Survival, and Multinucleated-Giant-Cell-Forming Activity of Burkholderia pseudomallei in Human Primary Phagocytic and Nonphagocytic Cells
- PMID: 28760929
- PMCID: PMC5607410
- DOI: 10.1128/IAI.00468-17
Entry, Intracellular Survival, and Multinucleated-Giant-Cell-Forming Activity of Burkholderia pseudomallei in Human Primary Phagocytic and Nonphagocytic Cells
Abstract
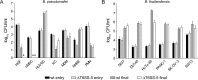
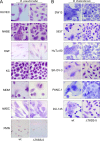
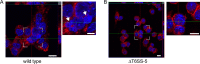
The human pathogen Burkholderia pseudomallei and the related species Burkholderia thailandensis are facultative intracellular bacteria characterized by the ability to escape into the cytosol of the host cell and to stimulate the formation of multinucleated giant cells (MNGCs). MNGC formation is induced via an unknown mechanism by bacterial type VI secretion system 5 (T6SS-5), which is an essential virulence factor in both species. Despite the vital role of the intracellular life cycle in the pathogenesis of the bacteria, the range of host cell types permissive for initiation and completion of the intracellular cycle is poorly defined. In the present study, we used several different types of human primary cells to evaluate bacterial entry, intracellular survival, and MNGC formation. We report the capacity of B. pseudomallei to enter, efficiently replicate in, and mediate MNGC formation of vein endothelial and bronchial epithelial cells, indicating that the T6SS-5 is important in the host-pathogen interaction in these cells. Furthermore, we show that B. pseudomallei invades fibroblasts and keratinocytes and survives inside these cells as well as in monocyte-derived macrophages and neutrophils for at least 17 h postinfection; however, MNGC formation is not induced in these cells. In contrast, infection of mixed neutrophils and RAW264.7 macrophages with B. thailandensis stimulated the formation of heterotypic MNGCs in a T6SS-5-dependent manner. In summary, the ability of the bacteria to enter and survive as well as induce MNGC formation in certain host cells may contribute to the pathogenesis observed in B. pseudomallei infection.
Keywords: Burkholderia pseudomallei; primary cells; type VI secretion system.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
A high-content imaging assay for the quantification of the Burkholderia pseudomallei induced multinucleated giant cell (MNGC) phenotype in murine macrophages.BMC Microbiol. 2014 Apr 22;14:98. doi: 10.1186/1471-2180-14-98. BMC Microbiol. 2014. PMID: 24750902 Free PMC article.
-
Burkholderia pseudomallei BicA protein promotes pathogenicity in macrophages by regulating invasion, intracellular survival, and virulence.mSphere. 2023 Oct 24;8(5):e0037823. doi: 10.1128/msphere.00378-23. Epub 2023 Sep 28. mSphere. 2023. PMID: 37768049 Free PMC article.
-
A role for tetraspanin proteins in regulating fusion induced by Burkholderia thailandensis.Med Microbiol Immunol. 2020 Aug;209(4):473-487. doi: 10.1007/s00430-020-00670-6. Epub 2020 Apr 6. Med Microbiol Immunol. 2020. PMID: 32253503 Free PMC article.
-
Intracellular replication of the well-armed pathogen Burkholderia pseudomallei.Curr Opin Microbiol. 2016 Feb;29:94-103. doi: 10.1016/j.mib.2015.11.007. Epub 2016 Jan 22. Curr Opin Microbiol. 2016. PMID: 26803404 Review.
-
The Burkholderia Type VI Secretion System 5: Composition, Regulation and Role in Virulence.Front Microbiol. 2019 Jan 10;9:3339. doi: 10.3389/fmicb.2018.03339. eCollection 2018. Front Microbiol. 2019. PMID: 30687298 Free PMC article. Review.
Cited by
-
Lactoferrin is a dynamic protein in human melioidosis and is a TLR4-dependent driver of TNF-α release in Burkholderia thailandensis infection in vitro.PLoS Negl Trop Dis. 2020 Aug 7;14(8):e0008495. doi: 10.1371/journal.pntd.0008495. eCollection 2020 Aug. PLoS Negl Trop Dis. 2020. PMID: 32764765 Free PMC article.
-
Burkholderia pseudomallei and melioidosis.Nat Rev Microbiol. 2024 Mar;22(3):155-169. doi: 10.1038/s41579-023-00972-5. Epub 2023 Oct 4. Nat Rev Microbiol. 2024. PMID: 37794173 Review.
-
Guanylate-Binding Protein-Dependent Noncanonical Inflammasome Activation Prevents Burkholderia thailandensis-Induced Multinucleated Giant Cell Formation.mBio. 2021 Aug 31;12(4):e0205421. doi: 10.1128/mBio.02054-21. Epub 2021 Aug 17. mBio. 2021. PMID: 34399626 Free PMC article.
-
RegAB Homolog of Burkholderia pseudomallei is the Master Regulator of Redox Control and involved in Virulence.PLoS Pathog. 2021 May 28;17(5):e1009604. doi: 10.1371/journal.ppat.1009604. eCollection 2021 May. PLoS Pathog. 2021. PMID: 34048488 Free PMC article.
-
Burkholderia pseudomallei pathogenesis in human skin fibroblasts: A Bsa type III secretion system is involved in the invasion, multinucleated giant cell formation, and cellular damage.PLoS One. 2022 Feb 3;17(2):e0261961. doi: 10.1371/journal.pone.0261961. eCollection 2022. PLoS One. 2022. PMID: 35113856 Free PMC article.
References
-
- Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NP, Peacock SJ, Hay SI. 2016. Predicted global distribution of and burden of melioidosis. Nat Microbiol 1(1):15008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

