Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia
- PMID: 28759039
- PMCID: PMC5690879
- DOI: 10.1038/onc.2017.247
Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia
Abstract
Obesity is associated with an increase in cancer-specific mortality in women with breast cancer. Elevated cholesterol, particularly low-density lipoprotein cholesterol (LDL-C), is frequently seen in obese women. Here, we aimed to determine the importance of elevated circulating LDL, and LDL receptor (LDLR) expression in tumor cells, on the growth of breast cancer using mouse models of hyperlipidemia. We describe two novel immunodeficient mouse models of hyperlipidemia (Rag1-/-/LDLR-/- and Rag1-/-/ApoE (apolipoprotein E)-/- mice) in addition to established immunocompetent LDLR-/- and ApoE-/- mice. The mice were used to study the effects of elevated LDL-C in human triple-negative (MDA-MB-231) and mouse Her2/Neu-overexpressing (MCNeuA) breast cancers. Tumors derived from MCNeuA and MDA-MB-231 cells had high LDLR expression and formed larger tumors in mice with high circulating LDL-C concentrations than in mice with lower LDL-C. Silencing the LDLR in the tumor cells led to decreased growth of Her2/Neu-overexpressing tumors in LDLR-/- and ApoE-/- mice, with increased Caspase 3 cleavage. Additionally, in vitro, silencing the LDLR led to decreased cell survival in serum-starved conditions, associated with Caspase 3 cleavage. Examining publically available human data sets, we found that high LDLR expression in human breast cancers was associated with decreased recurrence-free survival, particularly in patients treated with systemic therapies. Overall, our results highlight the importance of the LDLR in the growth of triple-negative and HER2-overexpressing breast cancers in the setting of elevated circulating LDL-C, which may be important contributing factors to the increased recurrence and mortality in obese women with breast cancer.
Conflict of interest statement
Figures
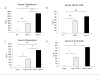
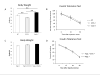





Similar articles
-
Cholesterol-induced mammary tumorigenesis is enhanced by adiponectin deficiency: role of LDL receptor upregulation.Oncotarget. 2013 Oct;4(10):1804-18. doi: 10.18632/oncotarget.1364. Oncotarget. 2013. PMID: 24113220 Free PMC article.
-
Regulation of low-density lipoprotein receptor expression in triple negative breast cancer by EGFR-MAPK signaling.Sci Rep. 2021 Sep 9;11(1):17927. doi: 10.1038/s41598-021-97327-y. Sci Rep. 2021. PMID: 34504181 Free PMC article.
-
Generation and characterization of two novel mouse models exhibiting the phenotypes of the metabolic syndrome: Apob48-/-Lepob/ob mice devoid of ApoE or Ldlr.Am J Physiol Endocrinol Metab. 2008 Mar;294(3):E496-505. doi: 10.1152/ajpendo.00509.2007. Epub 2007 Dec 26. Am J Physiol Endocrinol Metab. 2008. PMID: 18160459
-
Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoe and ldlr mice.Clin Chem Lab Med. 2005;43(5):470-9. doi: 10.1515/CCLM.2005.085. Clin Chem Lab Med. 2005. PMID: 15899668 Review.
-
General keynote: expression of epidermal growth factor receptor family in transgenic mouse models of human breast cancer.Gynecol Oncol. 2003 Jan;88(1 Pt 2):S43-6; discussion S52-5. doi: 10.1006/gyno.2002.6682. Gynecol Oncol. 2003. PMID: 12586084 Review. No abstract available.
Cited by
-
Metabolism: an important player in glioma survival and development.Discov Oncol. 2024 Oct 22;15(1):577. doi: 10.1007/s12672-024-01402-5. Discov Oncol. 2024. PMID: 39436434 Free PMC article. Review.
-
Lipoproteins and cancer: The role of HDL-C, LDL-C, and cholesterol-lowering drugs.Biochem Pharmacol. 2022 Feb;196:114654. doi: 10.1016/j.bcp.2021.114654. Epub 2021 Jun 12. Biochem Pharmacol. 2022. PMID: 34129857 Free PMC article. Review.
-
Metformin exhibited anticancer activity by lowering cellular cholesterol content in breast cancer cells.PLoS One. 2019 Jan 9;14(1):e0209435. doi: 10.1371/journal.pone.0209435. eCollection 2019. PLoS One. 2019. PMID: 30625181 Free PMC article.
-
Diabetes, Obesity, and Breast Cancer.Endocrinology. 2018 Nov 1;159(11):3801-3812. doi: 10.1210/en.2018-00574. Endocrinology. 2018. PMID: 30215698 Free PMC article. Review.
-
Cholesterol reprograms glucose and lipid metabolism to promote proliferation in colon cancer cells.Cancer Metab. 2023 Sep 13;11(1):15. doi: 10.1186/s40170-023-00315-1. Cancer Metab. 2023. PMID: 37705114 Free PMC article.
References
-
- Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, et al. The role of obesity in cancer survival and recurrence. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(8):1244–59. doi: 10.1158/1055-9965.EPI-12-0485. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

