Kaposi's sarcoma-associated herpesvirus infection promotes differentiation and polarization of monocytes into tumor-associated macrophages
- PMID: 28750175
- PMCID: PMC5587027
- DOI: 10.1080/15384101.2017.1356509
Kaposi's sarcoma-associated herpesvirus infection promotes differentiation and polarization of monocytes into tumor-associated macrophages
Abstract
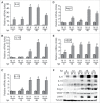
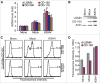
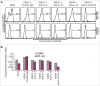
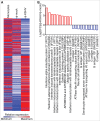
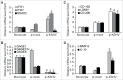
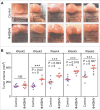
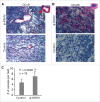
Tumor associated macrophages (TAMs) promote angiogenesis, tumor invasion and metastasis, and suppression of anti-tumor immunity. These myeloid cells originate from monocytes, which differentiate into TAMs upon exposure to the local tumor microenvironment. We previously reported that Kaposi's sarcoma-associated herpes virus (KSHV) infection of endothelial cells induces the cytokine angiopoietin-2 (Ang-2) to promote migration of monocytes into tumors. Here we report that KSHV infection of endothelial cells induces additional cytokines including interleukin-6 (IL-6), interleukin-10 (IL-10), and interleukin-13 (IL-13) that drive monocytes to differentiate and polarize into TAMs. The KSHV-induced TAMs not only express TAM-specific markers such as CD-163 and legumain (LGMN) but also display a gene expression profile with characteristic features of viral infection. More importantly, KSHV-induced TAMs enhance tumor growth in nude mice. These results are consistent with the strong presence of TAMs in Kaposi's sarcoma (KS) tumors. Therefore, KSHV infection of endothelial cells generates a local microenvironment that not only promotes the recruitment of monocytes but also induces their differentiation and polarization into TAMs. These findings reveal a new mechanism of KSHV contribution to KS tumor development.
Keywords: KSHV; Kaposi's sarcoma; monocytes; tumor-associated macrophages.
Figures

Similar articles
-
Suppression of KSHV-induced angiopoietin-2 inhibits angiogenesis, infiltration of inflammatory cells, and tumor growth.Cell Cycle. 2016 Aug 2;15(15):2053-65. doi: 10.1080/15384101.2016.1196303. Epub 2016 Jun 13. Cell Cycle. 2016. PMID: 27294705 Free PMC article.
-
KSHV induces aerobic glycolysis and angiogenesis through HIF-1-dependent upregulation of pyruvate kinase 2 in Kaposi's sarcoma.Angiogenesis. 2015 Oct;18(4):477-88. doi: 10.1007/s10456-015-9475-4. Epub 2015 Jun 20. Angiogenesis. 2015. PMID: 26092770 Free PMC article.
-
Role of heme oxygenase-1 in the pathogenesis and tumorigenicity of Kaposi's sarcoma-associated herpesvirus.Oncotarget. 2016 Mar 1;7(9):10459-71. doi: 10.18632/oncotarget.7227. Oncotarget. 2016. PMID: 26859574 Free PMC article.
-
Pathological Features of Kaposi's Sarcoma-Associated Herpesvirus Infection.Adv Exp Med Biol. 2018;1045:357-376. doi: 10.1007/978-981-10-7230-7_16. Adv Exp Med Biol. 2018. PMID: 29896675 Review.
-
Cellular origin of Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus-induced cell reprogramming.Trends Cell Biol. 2013 Sep;23(9):421-32. doi: 10.1016/j.tcb.2013.04.001. Epub 2013 May 17. Trends Cell Biol. 2013. PMID: 23685018 Review.
Cited by
-
Macrophages drive KSHV B cell latency.Cell Rep. 2023 Jul 25;42(7):112767. doi: 10.1016/j.celrep.2023.112767. Epub 2023 Jul 12. Cell Rep. 2023. PMID: 37440412 Free PMC article.
-
Therapeutic Approaches Targeting Proteins in Tumor-Associated Macrophages and Their Applications in Cancers.Biomolecules. 2022 Mar 2;12(3):392. doi: 10.3390/biom12030392. Biomolecules. 2022. PMID: 35327584 Free PMC article. Review.
-
Virally encoded interleukin-6 facilitates KSHV replication in monocytes and induction of dysfunctional macrophages.PLoS Pathog. 2023 Oct 26;19(10):e1011703. doi: 10.1371/journal.ppat.1011703. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37883374 Free PMC article.
-
Heterogeneous macrophages: Supersensors of exogenous inducing factors.Scand J Immunol. 2019 Jul;90(1):e12768. doi: 10.1111/sji.12768. Epub 2019 May 9. Scand J Immunol. 2019. PMID: 31002413 Free PMC article. Review.
-
Kaposi Sarcoma, a Trifecta of Pathogenic Mechanisms.Diagnostics (Basel). 2022 May 16;12(5):1242. doi: 10.3390/diagnostics12051242. Diagnostics (Basel). 2022. PMID: 35626397 Free PMC article. Review.
References
-
- Simonart T, Degraef C, Mosselmans R, Hermans P, Lunardi-Iskandar Y, Noel JC, Van Vooren JP, Parent D, Heenen M, Galand P. Early- and late-stage Kaposi's sarcoma-derived cells but not activated endothelial cells can invade de-epidermized dermis. J Invest Dermatol. 2001;116:679-85; http//dx.doi.org/10.1046/j.0022-202x.2001.doc.x. PMID:11348455. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
