Association of Risk of Suicide Attempts With Methylphenidate Treatment
- PMID: 28746699
- PMCID: PMC5710471
- DOI: 10.1001/jamapsychiatry.2017.2183
Association of Risk of Suicide Attempts With Methylphenidate Treatment
Abstract
Importance: Patients with attention-deficit/hyperactivity disorder (ADHD) are at an increased risk of attempting suicide. Stimulants, such as methylphenidate hydrochloride, are the most common treatment for ADHD, but the association between their therapeutic use and suicide is unclear.
Objective: To investigate the association between methylphenidate and the risk of suicide attempts.
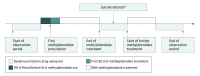
Design, setting, and participants: A population-based, electronic medical records database from the Hong Kong Clinical Data Analysis & Reporting System was used to identify 25 629 individuals aged 6 to 25 years who were treated with methylphenidate between January 1, 2001, and December 31, 2015. Those who had attempted suicide were included in the analysis. A self-controlled case series design was used to control for time-invariant characteristics of the patients.
Main outcomes and measures: Relative incidence of suicide attempt during periods when patients were exposed to methylphenidate compared with nonexposed periods.
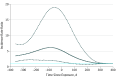
Results: Among 25 629 patients with methylphenidate prescriptions, 154 had their first recorded suicide attempt within the study period; of these individuals, 111 (72.1%) were male; mean (SD) age at baseline was 7.15 (2.19) years. The overall incidence of suicide attempts during methylphenidate treatment was 9.27 per 10 000 patient-years. An increased risk of suicide attempts was detected during the 90-day period before methylphenidate was initiated, with an incidence rate ratio (IRR) of 6.55 (95% CI, 3.37-12.72). The IRR remained elevated during the first 90 days of treatment (IRR, 3.91; 95% CI, 1.62-9.42) before returning to baseline levels during ongoing treatment (IRR, 1.35; 95% CI, 0.77-2.38). When the risk during the first 90 days of treatment was compared with the 90 days preceding first treatment, the incidence of suicide attempts was not elevated (IRR, 0.78; 95% CI, 0.26-2.35).
Conclusions and relevance: The incidence of suicide attempts was higher in the period immediately before the start of methylphenidate treatment. The risk remained elevated immediately after the start of methylphenidate treatment and returned to baseline levels during continuation of methylphenidate treatment. The observed higher risk of suicide attempts before treatment may reflect emerging psychiatric symptoms that trigger medical consultations that result in a decision to begin ADHD treatment. Therefore, this study's results do not support a causal association between methylphenidate treatment and suicide attempts.
Conflict of interest statement
Figures
Similar articles
-
Association between methylphenidate treatment and risk of seizure: a population-based, self-controlled case-series study.Lancet Child Adolesc Health. 2020 Jun;4(6):435-443. doi: 10.1016/S2352-4642(20)30100-0. Lancet Child Adolesc Health. 2020. PMID: 32450123
-
Suicide risk reduction in youths with attention-deficit/hyperactivity disorder prescribed methylphenidate: A Taiwan nationwide population-based cohort study.Res Dev Disabil. 2018 Jan;72:96-105. doi: 10.1016/j.ridd.2017.10.023. Epub 2017 Nov 6. Res Dev Disabil. 2018. PMID: 29121517
-
Treatment with Methylphenidate for Attention Deficit Hyperactivity Disorder (ADHD) and the Risk of All-Cause Poisoning in Children and Adolescents: A Self-Controlled Case Series Study.CNS Drugs. 2021 Jul;35(7):769-779. doi: 10.1007/s40263-021-00824-x. Epub 2021 Jul 20. CNS Drugs. 2021. PMID: 34283391 Free PMC article.
-
Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents - assessment of adverse events in non-randomised studies.Cochrane Database Syst Rev. 2018 May 9;5(5):CD012069. doi: 10.1002/14651858.CD012069.pub2. Cochrane Database Syst Rev. 2018. PMID: 29744873 Free PMC article. Review.
-
Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults.Cochrane Database Syst Rev. 2014 Sep 18;(9):CD005041. doi: 10.1002/14651858.CD005041.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2016 May 26;(5):CD005041. doi: 10.1002/14651858.CD005041.pub3 PMID: 25230710 Updated. Review.
Cited by
-
Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan.Eur Neuropsychopharmacol. 2018 Oct;28(10):1059-1088. doi: 10.1016/j.euroneuro.2018.08.001. Epub 2018 Sep 6. Eur Neuropsychopharmacol. 2018. PMID: 30195575 Free PMC article. Review.
-
Associations between childhood maltreatment and psychiatric disorders: analysis from electronic health records in Hong Kong.Transl Psychiatry. 2022 Jun 6;12(1):231. doi: 10.1038/s41398-022-01986-y. Transl Psychiatry. 2022. PMID: 35668084 Free PMC article.
-
Application of Healthcare 'Big Data' in CNS Drug Research: The Example of the Neurological and mental health Global Epidemiology Network (NeuroGEN).CNS Drugs. 2020 Sep;34(9):897-913. doi: 10.1007/s40263-020-00742-4. CNS Drugs. 2020. PMID: 32572794 Free PMC article.
-
Medication for Attention-Deficit/Hyperactivity Disorder and Risk for Suicide Attempts.Biol Psychiatry. 2020 Sep 15;88(6):452-458. doi: 10.1016/j.biopsych.2019.12.003. Epub 2019 Dec 13. Biol Psychiatry. 2020. PMID: 31987492 Free PMC article.
-
Bipolar disorder prevalence and psychotropic medication utilisation in Hong Kong and the United Kingdom.Pharmacoepidemiol Drug Saf. 2021 Nov;30(11):1588-1600. doi: 10.1002/pds.5318. Epub 2021 Jul 8. Pharmacoepidemiol Drug Saf. 2021. PMID: 34180569 Free PMC article.
References
-
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994-e1001. - PubMed
-
- Leung PW, Luk SL, Ho TP, Taylor E, Mak FL, Bacon-Shone J. The diagnosis and prevalence of hyperactivity in Chinese schoolboys. Br J Psychiatry. 1996;168(4):486-496. - PubMed
-
- Leung PW, Hung SF, Ho TP, et al. . Prevalence of DSM-IV disorders in Chinese adolescents and the effects of an impairment criterion: a pilot community study in Hong Kong. Eur Child Adolesc Psychiatry. 2008;17(7):452-461. - PubMed
-
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

