CCR6 Deficiency Impairs IgA Production and Dysregulates Antimicrobial Peptide Production, Altering the Intestinal Flora
- PMID: 28744287
- PMCID: PMC5504188
- DOI: 10.3389/fimmu.2017.00805
CCR6 Deficiency Impairs IgA Production and Dysregulates Antimicrobial Peptide Production, Altering the Intestinal Flora
Abstract
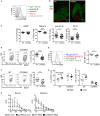
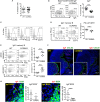
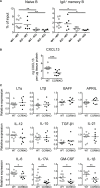
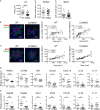
Intestinal immunity exists as a complex relationship among immune cells, epithelial cells, and microbiota. CCR6 and its ligand-CCL20 are highly expressed in intestinal mucosal tissues, such as Peyer's patches (PPs) and isolated lymphoid follicles (ILFs). In this study, we investigated the role of the CCR6-CCL20 axis in intestinal immunity under homeostatic conditions. CCR6 deficiency intrinsically affects germinal center reactions in PPs, leading to impairments in IgA class switching, IgA affinity, and IgA memory B cell production and positioning in PPs, suggesting an important role for CCR6 in T-cell-dependent IgA generation. CCR6 deficiency impairs the maturation of ILFs. In these follicles, group 3 innate lymphoid cells are important components and a major source of IL-22, which stimulates intestinal epithelial cells (IECs) to produce antimicrobial peptides (AMPs). We found that CCR6 deficiency reduces IL-22 production, likely due to diminished numbers of group 3 innate lymphoid cells within small-sized ILFs. The reduced IL-22 levels subsequently decrease the production of AMPs, suggesting a critical role for CCR6 in innate intestinal immunity. Finally, we found that CCR6 deficiency impairs the production of IgA and AMPs, leading to increased levels of Alcaligenes in PPs, and segmented filamentous bacteria in IECs. Thus, the CCR6-CCL20 axis plays a crucial role in maintaining intestinal symbiosis by limiting the overgrowth of mucosa-associated commensal bacteria.
Keywords: CCR6; IgA; Peyer’s patch; antimicrobial peptide; isolated lymphoid follicle.
Figures





Similar articles
-
CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis.Ann N Y Acad Sci. 2006 Aug;1072:52-61. doi: 10.1196/annals.1326.036. Ann N Y Acad Sci. 2006. PMID: 17057190 Review.
-
CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles.Am J Pathol. 2007 Apr;170(4):1229-40. doi: 10.2353/ajpath.2007.060817. Am J Pathol. 2007. PMID: 17392163 Free PMC article.
-
Re-thinking the functions of IgA(+) plasma cells.Gut Microbes. 2014;5(5):652-62. doi: 10.4161/19490976.2014.969977. Gut Microbes. 2014. PMID: 25483334 Free PMC article. Review.
-
Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis.Nature. 2008 Nov 27;456(7221):507-10. doi: 10.1038/nature07450. Epub 2008 Nov 5. Nature. 2008. PMID: 18987631
-
Absence of CCR6 inhibits CD4+ regulatory T-cell development and M-cell formation inside Peyer's patches.Am J Pathol. 2005 Jun;166(6):1647-54. doi: 10.1016/S0002-9440(10)62475-3. Am J Pathol. 2005. PMID: 15920150 Free PMC article.
Cited by
-
IL-22: An Underestimated Player in Natural Resistance to Tuberculosis?Front Immunol. 2018 Sep 25;9:2209. doi: 10.3389/fimmu.2018.02209. eCollection 2018. Front Immunol. 2018. PMID: 30319650 Free PMC article. Review.
-
The Phosphorylation of CCR6 on Distinct Ser/Thr Residues in the Carboxyl Terminus Differentially Regulates Biological Function.Front Immunol. 2018 Mar 2;9:415. doi: 10.3389/fimmu.2018.00415. eCollection 2018. Front Immunol. 2018. PMID: 29552015 Free PMC article.
-
Atypical chemokine receptors in the immune system.Nat Rev Immunol. 2024 Oct;24(10):753-769. doi: 10.1038/s41577-024-01025-5. Epub 2024 May 7. Nat Rev Immunol. 2024. PMID: 38714818 Review.
-
Galleria mellonella: A Novel Invertebrate Model to Distinguish Intestinal Symbionts From Pathobionts.Front Immunol. 2018 Sep 19;9:2114. doi: 10.3389/fimmu.2018.02114. eCollection 2018. Front Immunol. 2018. PMID: 30283451 Free PMC article.
-
Lymphoid tissue inducer-A divergent member of the ILC family.Cytokine Growth Factor Rev. 2018 Aug;42:5-12. doi: 10.1016/j.cytogfr.2018.02.004. Epub 2018 Feb 13. Cytokine Growth Factor Rev. 2018. PMID: 29454785 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

